Evaluation of electroacupuncture as a non-pharmacological therapy for astrocytic structural aberrations and behavioral deficits in a post-ischemic depression model in mice
- PMID: 37700911
- PMCID: PMC10493307
- DOI: 10.3389/fnbeh.2023.1239024
Evaluation of electroacupuncture as a non-pharmacological therapy for astrocytic structural aberrations and behavioral deficits in a post-ischemic depression model in mice
Abstract
Background: Ascending clinical evidence supports that electroacupuncture (EA) is effective in treating post-ischemic depression (PID), but little is known about how it works at the cellular level. Astrocytes are exquisitely sensitive to their extracellular environment, and under stressful conditions, they may experience aberrant structural remodeling that can potentially cause neuroplastic disturbances and contribute to subsequent changes in mood or behavior.
Objectives: This study aimed to investigate the effect of EA on behavioral deficits associated with PID in mice and verify the hypothesis that astrocytic morphology may be involved in this impact.
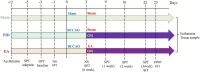
Methods: We established a PID animal model induced by transient bilateral common carotid artery occlusion (BCCAO, 20 min) and chronic restraint stress (CRS, 21 days). EA treatment (GV20 + ST36) was performed for 3 weeks, from Monday to Friday each week. Depressive- and anxiety-like behaviors and sociability were evaluated using SPT, FST, EPM, and SIT. Immunohistochemistry combined with Sholl and cell morphological analysis was utilized to assess the process morphology of GFAP+ astrocytes in mood-related regions. The potential relationship between morphological changes in astrocytes and behavioral output was detected by correlation analysis.
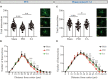
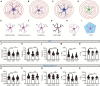
Results: Behavioral assays demonstrated that EA treatment induced an overall reduction in behavioral deficits, as measured by the behavioral Z-score. Sholl and morphological analyses revealed that EA prevented the decline in cell complexity of astrocytes in the prefrontal cortex (PFC) and the CA1 region of the hippocampus, where astrocytes displayed evident deramification and atrophy of the branches. Eventually, the correlation analysis showed there was a relationship between behavioral emotionality and morphological changes.
Conclusion: Our findings imply that EA prevents both behavioral deficits and structural abnormalities in astrocytes in the PID model. The strong correlation between behavioral Z-scores and the observed morphological changes confirms the notion that the weakening of astrocytic processes may play a crucial role in depressive symptoms, and astrocytes could be a potential target of EA in the treatment of PID.
Keywords: BCCAO; astrocyte; bilateral common carotid artery occlusion; branch morphology; depressive-like behavior; electroacupuncture; post-ischemic depression; stress.
Copyright © 2023 Wang, Deng, Jiang, Yao, Ju and Luo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Althammer F., Ferreira-Neto H. C., Rubaharan M., Roy R. K., Patel A. A., Murphy A., et al. (2020). Three-dimensional morphometric analysis reveals time-dependent structural changes in microglia and astrocytes in the central amygdala and hypothalamic paraventricular nucleus of heart failure rats. J. Neuroinflamm. 17:221. 10.1186/s12974-020-01892-4 - DOI - PMC - PubMed
-
- Anderson R. M., Johnson S. B., Lingg R. T., Hinz D. C., Romig-Martin S. A., Radley J. J. (2020). Evidence for similar prefrontal structural and functional alterations in male and female rats following chronic stress or glucocorticoid exposure. Cereb. Cortex 30 353–370. 10.1093/cercor/bhz092 - DOI - PMC - PubMed
-
- Cai W., Ma W., Wang G. T., Li Y. J., Shen W. D. (2019). Antidepressant, anti-inflammatory, and antioxidant effects of electroacupuncture through sonic hedgehog-signaling pathway in a rat model of poststroke depression. Neuropsychiatr. Dis. Treat. 15 1403–1411. 10.2147/NDT.S205033 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

