Lumbar V3 interneurons provide direct excitatory synaptic input onto thoracic sympathetic preganglionic neurons, linking locomotor, and autonomic spinal systems
- PMID: 37701071
- PMCID: PMC10493276
- DOI: 10.3389/fncir.2023.1235181
Lumbar V3 interneurons provide direct excitatory synaptic input onto thoracic sympathetic preganglionic neurons, linking locomotor, and autonomic spinal systems
Abstract
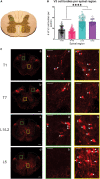
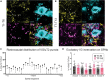
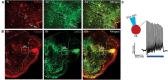
Although sympathetic autonomic systems are activated in parallel with locomotion, the neural mechanisms mediating this coordination are incompletely understood. Sympathetic preganglionic neurons (SPNs), primarily located in the intermediate laminae of thoracic and upper lumbar segments (T1-L2), increase activation of tissues and organs that provide homeostatic and metabolic support during movement and exercise. Recent evidence suggests integration between locomotor and autonomic nuclei occurs within the brainstem, initiating both descending locomotor and sympathetic activation commands. However, both locomotor and sympathetic autonomic spinal systems can be activated independent of supraspinal input, in part due to a distributed network involving propriospinal neurons. Whether an intraspinal mechanism exists to coordinate activation of these systems is unknown. We hypothesized that ascending spinal neurons located in the lumbar region provide synaptic input to thoracic SPNs. Here, we demonstrate that synaptic contacts from locomotor-related V3 interneurons (INs) are present in all thoracic laminae. Injection of an anterograde tracer into lumbar segments demonstrated that 8-20% of glutamatergic input onto SPNs originated from lumbar V3 INs and displayed a somatotopographical organization of synaptic input. Whole cell patch clamp recording in SPNs demonstrated prolonged depolarizations or action potentials in response to optical activation of either lumbar V3 INs in spinal cord preparations or in response to optical activation of V3 terminals in thoracic slice preparations. This work demonstrates a direct intraspinal connection between lumbar locomotor and thoracic sympathetic networks and suggests communication between motor and autonomic systems may be a general function of the spinal cord.
Keywords: motor systems; optical stimulation; propriospinal neurons; spinal interneurons; sympathetic preganglionic neurons.
Copyright © 2023 Chacon, Nwachukwu, Shahsavani, Cowley and Chopek.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Enkephalin-immunoreactive interneurons extensively innervate sympathetic preganglionic neurons regulating the pelvic viscera.J Comp Neurol. 2005 Aug 1;488(3):278-89. doi: 10.1002/cne.20552. J Comp Neurol. 2005. PMID: 15952166
-
Field potential mapping of neurons in the lumbar spinal cord activated following stimulation of the mesencephalic locomotor region.J Neurosci. 1995 Mar;15(3 Pt 2):2203-17. doi: 10.1523/JNEUROSCI.15-03-02203.1995. J Neurosci. 1995. PMID: 7891162 Free PMC article.
-
Origin of thoracic spinal network activity during locomotor-like activity in the neonatal rat.J Neurosci. 2015 Apr 15;35(15):6117-30. doi: 10.1523/JNEUROSCI.4145-14.2015. J Neurosci. 2015. PMID: 25878284 Free PMC article.
-
A new conceptual framework for the integrated neural control of locomotor and sympathetic function: implications for exercise after spinal cord injury.Appl Physiol Nutr Metab. 2018 Nov;43(11):1140-1150. doi: 10.1139/apnm-2018-0310. Appl Physiol Nutr Metab. 2018. PMID: 30071179 Review.
-
Effects of spinal cord injury on synaptic inputs to sympathetic preganglionic neurons.Prog Brain Res. 2006;152:11-26. doi: 10.1016/S0079-6123(05)52001-6. Prog Brain Res. 2006. PMID: 16198690 Review.
Cited by
-
Propriospinal myoclonus following cervical spinal cord injury: a case report and mechanistic insights.J Neurol. 2025 Jan 15;272(2):126. doi: 10.1007/s00415-024-12880-6. J Neurol. 2025. PMID: 39812827 Free PMC article. No abstract available.
-
Sacral Bioneuromodulation: The Role of Bone Marrow Aspirate in Spinal Cord Injuries.Bioengineering (Basel). 2024 May 6;11(5):461. doi: 10.3390/bioengineering11050461. Bioengineering (Basel). 2024. PMID: 38790327 Free PMC article. Review.
References
-
- Adler E. S., Hollis J. H., I, Clarke J., Grattan D. R., Oldfield B. J. (2012). Neurochemical characterization and sexual dimorphism of projections from the brain to abdominal and subcutaneous white adipose tissue in the rat. J. Neurosci. 32 15913–15921. 10.1523/JNEUROSCI.2591-12.2012 - DOI - PMC - PubMed
-
- Armstrong K. E., Nazzal M., Chen X., Stecina K., Jordan L. M. (2017). “Chemogenic activation of parapyramidal brainstem neurons to evaluate motor consequences,” in Society for neuroscience abstracts, (Washington, DC: Society for Neuroscience; ). Available online at: http://www.abstractsonline.com/pp8/#!/4376/presentation/4276 (accessed May 9, 2018).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

