Identification of two terpenoids that accumulate in Chinese water chestnut in response to fresh-cut processing
- PMID: 37701225
- PMCID: PMC10494652
- DOI: 10.1002/fsn3.3475
Identification of two terpenoids that accumulate in Chinese water chestnut in response to fresh-cut processing
Abstract
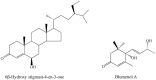
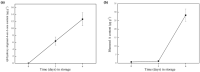
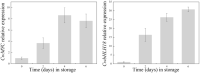
As a form of vegetable in China, freshly cut corms of Chinese water chestnuts (Eleocharis dulcis) are well received by consumers. Few studies have investigated the metabolites present in fresh-cut E. dulcis, particularly during the storage stage. Two compounds, triterpenoids and apocarotenoids, were identified in fresh-cut E. dulcis during the late storage period using thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), and nuclear magnetic resonance (NMR) spectroscopy. The content of these two compounds gradually increased in the surface tissue of fresh-cut E. dulcis during storage. Moreover, the transcript levels of 10 genes involved in terpenoid backbone biosynthesis and five genes involved in carotenoid precursor biosynthesis were evaluated via quantitative real-time PCR (qRT-PCR). Expression of the rate-limiting enzyme-coding genes CwDXS and CwHMGS was significantly induced by wounding. CwMYC and CwbHLH18, which belong to bHLH transcription factors (TFs) IIIe and VIa subgroup, were isolated from E. dulcis corm. Phylogenetic analysis showed that CwMYC and CwbHLH18 grouped with other terpenoid-regulated bHLHs, and their transcript levels were strongly induced after fresh-cut processing. These results suggested that the biosynthesis of terpenoids and apocarotenoids in fresh-cut E. dulcis strongly depended on the transcriptional regulation of structural genes involved in the methylerythritol 4-phosphate (MEP) and mevalonate (MVA) pathways. However, the complex secondary metabolism of fresh-cut E. dulcis during late storage requires further investigation.
Keywords: Chinese water chestnut; purification; secondary metabolism; structural identification; terpenoid biosynthesis.
© 2023 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

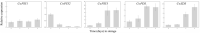
Similar articles
-
Isolation, purification and identification of etiolation substrate from fresh-cut Chinese water-chestnut (Eleocharis tuberosa).Food Chem. 2015 Nov 1;186:119-22. doi: 10.1016/j.foodchem.2015.03.070. Epub 2015 Mar 26. Food Chem. 2015. PMID: 25976800
-
Eleocharis dulcis corm: phytochemicals, health benefits, processing and food products.J Sci Food Agric. 2022 Jan 15;102(1):19-40. doi: 10.1002/jsfa.11508. Epub 2021 Sep 12. J Sci Food Agric. 2022. PMID: 34453323 Review.
-
Mechanism of exogenous methyl jasmonate in regulating the quality of fresh-cut Chinese water chestnuts.Front Plant Sci. 2024 Aug 15;15:1435066. doi: 10.3389/fpls.2024.1435066. eCollection 2024. Front Plant Sci. 2024. PMID: 39220004 Free PMC article.
-
Identification and characterization of water chestnut Soymovirus-1 (WCSV-1), a novel Soymovirus in water chestnuts (Eleocharis dulcis).BMC Plant Biol. 2019 Apr 25;19(1):159. doi: 10.1186/s12870-019-1761-7. BMC Plant Biol. 2019. PMID: 31023231 Free PMC article.
-
Biosynthesis of Phenolic Compounds and Antioxidant Activity in Fresh-Cut Fruits and Vegetables.Front Microbiol. 2022 May 25;13:906069. doi: 10.3389/fmicb.2022.906069. eCollection 2022. Front Microbiol. 2022. PMID: 35694311 Free PMC article. Review.
References
-
- Alfieri, M. , Vaccaro, M. C. , Cappetta, E. , Ambrosone, A. , De Tommasi, N. , & Leone, A. (2018). Coactivation of MEP‐biosynthetic genes and accumulation of abietane diterpenes in Salvia sclarea by heterologous expression of WRKY and MYC2 transcription factors. Scientific Reports, 8, 11009–11021. - PMC - PubMed
-
- Balusamy, S. R. D. , Rahimi, S. , Sukweenadhi, J. , Kim, Y. , & Yang, D. (2015). Exogenous methyl jasmonate prevents necrosis caused by mechanical wounding and increases terpenoid biosynthesis in Panax ginseng. Plant Cell Tissue and Organ Culture, 123, 341–348.
-
- Barrett, D. M. , Beaulieu, J. C. , & Shewfelt, R. (2010). Color, flavor, texture, and nutritional quality of fresh‐cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Critical Reviews in Food Science and Nutrition, 50, 369–389. - PubMed
-
- Bartram, S. , Jux, A. , Gleixner, G. , & Boland, W. (2006). Dynamic pathway allocation in early terpenoid biosynthesis of stress‐induced lima bean leaves. Phytochemistry, 67, 1661–1672. - PubMed
-
- Cazzonelli, C. I. , & Pogson, B. J. (2010). Source to sink: Regulation of carotenoid biosynthesis in plants. Trends in Plant Science, 15, 266–274. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

