The effect of celery (Apium graveolens) powder on cardiometabolic factors in overweight/obese individuals with type 2 diabetes mellitus: A pilot randomized, double-blinded, placebo-controlled clinical trial
- PMID: 37701242
- PMCID: PMC10494649
- DOI: 10.1002/fsn3.3493
The effect of celery (Apium graveolens) powder on cardiometabolic factors in overweight/obese individuals with type 2 diabetes mellitus: A pilot randomized, double-blinded, placebo-controlled clinical trial
Abstract
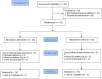
Celery (Apium graveolens) was shown to have beneficial effects on cardiometabolic factors in animal models. As the progression of type 2 diabetes mellitus (T2DM) adversely affects cardiometabolic factors, we aimed to assess the effects of celery powder on glycemic and anthropometric indices, lipid profile, liver function, oxidative stress, and blood pressure of individuals with T2DM. In a pilot randomized, double-blinded, placebo-controlled clinical trial, 50 eligible adults with T2DM were randomly divided into two groups of intervention and control to consume either 750 mg of celery powder (obtained from fresh celery) or placebo along with a low-calorie diet for 12 weeks, respectively. Dietary intake, physical activity, and cardiometabolic factors were assessed before and at the end of the study. Thirty-six patients finished the study (18 in each group). Consumption of celery powder significantly reduced body fat percentage (p = .021). Between-group analysis for changes in cardiometabolic factors did not show significant differences. Although malondialdehyde was reduced in the intervention group and increased in the control group, between-group changes were not significant. Although the insulin-level change was statistically insignificant, a clinical improvement was observed in the intervention group. A 750-mg daily supplementation of celery powder for 12 weeks did not improve the cardiometabolic factors of patients with T2DM. Further studies are suggested.
Keywords: Apium graveolens; celery; hyperglycemia; hyperlipidemia; hypertension; weight loss.
© 2023 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they do not have any conflict of interest.
Figures
Similar articles
-
Effect of celery (Apium graveolens) seed extract on hypertension: A randomized, triple-blind, placebo-controlled, cross-over, clinical trial.Phytother Res. 2022 Jul;36(7):2889-2907. doi: 10.1002/ptr.7469. Epub 2022 May 27. Phytother Res. 2022. PMID: 35624525 Clinical Trial.
-
Beneficial effects of celery (Apium graveolens) on metabolic syndrome: A review of the existing evidences.Phytother Res. 2019 Dec;33(12):3040-3053. doi: 10.1002/ptr.6492. Epub 2019 Aug 29. Phytother Res. 2019. PMID: 31464016 Review.
-
Beneficial impacts of fermented celery (Apium graveolens L.) juice on obesity prevention and gut microbiota modulation in high-fat diet fed mice.Food Funct. 2021 Oct 4;12(19):9151-9164. doi: 10.1039/d1fo00560j. Food Funct. 2021. PMID: 34606532
-
Safety evaluation and biochemical efficacy of celery seed extract (Apium Graveolens) capsules in hypertensive patients: a randomized, triple-blind, placebo-controlled, cross-over, clinical trial.Inflammopharmacology. 2022 Oct;30(5):1669-1684. doi: 10.1007/s10787-022-00986-0. Epub 2022 May 10. Inflammopharmacology. 2022. PMID: 35536382 Clinical Trial.
-
A Review of the Antioxidant Activity of Celery ( Apium graveolens L).J Evid Based Complementary Altern Med. 2017 Oct;22(4):1029-1034. doi: 10.1177/2156587217717415. Epub 2017 Jul 13. J Evid Based Complementary Altern Med. 2017. PMID: 28701046 Free PMC article. Review.
Cited by
-
Vegan gummy candies with low calorie based on celery (Apium graveolens) puree and boswellia gum (Boswellia thurifera).Food Sci Nutr. 2024 May 22;12(8):5785-5798. doi: 10.1002/fsn3.4190. eCollection 2024 Aug. Food Sci Nutr. 2024. PMID: 39139949 Free PMC article.
-
Effects of celery (Apium graveolens) on blood pressure, glycemic and lipid profile in adults: a systematic review and meta-analysis of randomized controlled trials.Front Nutr. 2025 Jul 22;12:1597680. doi: 10.3389/fnut.2025.1597680. eCollection 2025. Front Nutr. 2025. PMID: 40765738 Free PMC article.
-
The effect of chitosan supplementation on liver function, hepatic steatosis predictors, and metabolic indicators in adults with non-alcoholic fatty liver disease: a randomized, double-blinded, placebo-controlled, clinical trial.J Health Popul Nutr. 2025 Mar 1;44(1):60. doi: 10.1186/s41043-025-00797-3. J Health Popul Nutr. 2025. PMID: 40025618 Free PMC article. Clinical Trial.
References
-
- Abdollahi, S. , Salehi‐Abargouei, A. , Toupchian, O. , Sheikhha, M. H. , Fallahzadeh, H. , Rahmanian, M. , Tabatabaie, M. , & Mozaffari‐Khosravi, H. (2019). The effect of resveratrol supplementation on cardio‐metabolic risk factors in patients with type 2 diabetes: A randomized, double‐blind controlled trial. Phytotherapy Research, 33(12), 3153–3162. - PubMed
-
- Abshirini, M. , Siassi, F. , Koohdani, F. , Qorbani, M. , Mozaffari, H. , Aslani, Z. , Soleymani, M. , Entezarian, M. , & Sotoudeh, G. (2019). Dietary total antioxidant capacity is inversely associated with depression, anxiety and some oxidative stress biomarkers in postmenopausal women: A cross‐sectional study. Annals of General Psychiatry, 18(1), 1–9. - PMC - PubMed
-
- Carbone, S. , Del Buono, M. G. , Ozemek, C. , & Lavie, C. J. (2019). Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Progress in Cardiovascular Diseases, 62(4), 327–333. - PubMed
-
- Carrillo‐Larco, R. M. , Barengo, N. C. , Albitres‐Flores, L. , & Bernabe‐Ortiz, A. (2019). The risk of mortality among people with type 2 diabetes in Latin America: A systematic review and meta‐analysis of population‐based cohort studies. Diabetes/Metabolism Research and Reviews, 35(4), e3139. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials