Enhancing solubility and stability of sorafenib through cyclodextrin-based inclusion complexation: in silico and in vitro studies
- PMID: 37701271
- PMCID: PMC10494890
- DOI: 10.1039/d3ra03867j
Enhancing solubility and stability of sorafenib through cyclodextrin-based inclusion complexation: in silico and in vitro studies
Abstract
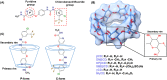
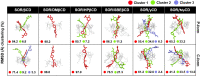
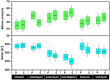
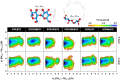
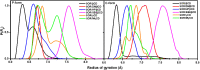
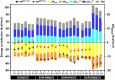
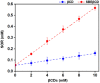
Sorafenib (SOR) is an oral multikinase inhibitor that effectively hampers the growth and spread of cancer cells by targeting angiogenesis and proliferation. However, SOR tablets (Nexavar) have limited oral bioavailability, ranging from 38% to 49%, due to their low water solubility. To address this issue, cyclodextrins (CDs), widely used to enhance the solubility and stability of lipophilic drugs by encapsulating them within their molecular structure, were considered in this study. We focused on β-cyclodextrin (βCD) and its derivatives, including hydroxypropyl-β-cyclodextrin (HPβCD), dimethyl-β-cyclodextrin (DMβCD), sulfobutylether-β-cyclodextrin (SBEβCD), and compared them with γ-cyclodextrin (γCD) for generating inclusion complexes with SOR. The 200 ns molecular dynamics simulations revealed that SOR could form inclusion complexes with all CDs in two possible orientations: pyridine group insertion (P-form) and chlorobenzotrifluoride group insertion (C-form), primarily driven by van der Waals interactions. Among the four βCD derivatives studied, SOR exhibited the highest number of atom contacts with SBEβCD and demonstrated the lowest solvent accessibility within the hydrophobic cavity of SBEβCD. These findings correlated with the highest binding affinity of SOR/SBEβCD complex determined by SIE, MM/GBSA, and MM/PBSA methods. Experimental results further supported our computational predictions, in which SBEβCD exhibited a stability constant of 940 M-1 at 25 °C, surpassing βCD's stability constant of 210 M-1. Taken together, our results suggest that the modified CDs, particularly SBEβCD, hold promising potential as an efficient molecular encapsulating agent for SOR, offering improved solubility and stability for this lipophilic drug.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors report no conflict of interest, financial or otherwise.
Figures




Similar articles
-
Evaluating solubility, stability, and inclusion complexation of oxyresveratrol with various β-cyclodextrin derivatives using advanced computational techniques and experimental validation.Comput Biol Chem. 2024 Oct;112:108111. doi: 10.1016/j.compbiolchem.2024.108111. Epub 2024 Jun 1. Comput Biol Chem. 2024. PMID: 38879954
-
Enhancing solubility and stability of piperine using β-cyclodextrin derivatives: computational and experimental investigations.J Biomol Struct Dyn. 2025 Mar;43(5):2596-2609. doi: 10.1080/07391102.2024.2305696. Epub 2024 Jan 23. J Biomol Struct Dyn. 2025. PMID: 38260962
-
SN-38-cyclodextrin complexation and its influence on the solubility, stability, and in vitro anticancer activity against ovarian cancer.AAPS PharmSciTech. 2014 Apr;15(2):472-82. doi: 10.1208/s12249-013-0068-5. Epub 2014 Jan 30. AAPS PharmSciTech. 2014. PMID: 24477982 Free PMC article.
-
Effect of beta-cyclodextrin and hydroxypropyl beta-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir.J Pharm Biomed Anal. 2008 Jul 15;47(3):535-40. doi: 10.1016/j.jpba.2008.02.006. Epub 2008 Feb 15. J Pharm Biomed Anal. 2008. PMID: 18367363 Review.
-
Self-assembled γ-cyclodextrin as nanocarriers for enhanced ocular drug bioavailability.Int J Pharm. 2022 Apr 25;618:121654. doi: 10.1016/j.ijpharm.2022.121654. Epub 2022 Mar 9. Int J Pharm. 2022. PMID: 35278603 Review.
Cited by
-
Encapsulation of Nanocrystals in Mannitol-Based Inhalable Microparticles via Spray-Drying: A Promising Strategy for Lung Delivery of Curcumin.Pharmaceuticals (Basel). 2024 Dec 18;17(12):1708. doi: 10.3390/ph17121708. Pharmaceuticals (Basel). 2024. PMID: 39770549 Free PMC article.
-
Essential oil-derived compounds target core fatigue-related genes: A network pharmacology and molecular Docking approach.PLoS One. 2025 May 28;20(5):e0314125. doi: 10.1371/journal.pone.0314125. eCollection 2025. PLoS One. 2025. PMID: 40435272 Free PMC article.
References
-
- Bondì M. L. Scala A. Sortino G. Amore E. Botto C. Azzolina A. Balasus D. Cervello M. Mazzaglia A. Biomacromolecules. 2015;16:3784–3791. - PubMed
-
- Wilhelm S. M. Carter C. Tang L. Wilkie D. McNabola A. Rong H. Chen C. Zhang X. Vincent P. McHugh M. Cao Y. Shujath J. Gawlak S. Eveleigh D. Rowley B. Liu L. Adnane L. Lynch M. Auclair D. Taylor I. Gedrich R. Voznesensky A. Riedl B. Post L. E. Bollag G. Trail P. A. Cancer Res. 2004;64:7099–7109. - PubMed
-
- Llovet J. M. Di Bisceglie A. M. Bruix J. Kramer B. S. Lencioni R. Zhu A. X. Sherman M. Schwartz M. Lotze M. Talwalkar J. Gores G. J. Panel of Experts in HCC D. C. T. J. Natl. Cancer Inst. 2008;100:698–711. - PubMed
-
- Wang X. Q. Fan J. M. Liu Y. O. Zhao B. Jia Z. R. Zhang Q. Int. J. Pharm. 2011;419:339–346. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

