Non-noble metal-catalyzed cross-dehydrogenation coupling (CDC) involving ether α-C(sp3)-H to construct C-C bonds
- PMID: 37701303
- PMCID: PMC10494247
- DOI: 10.3762/bjoc.19.94
Non-noble metal-catalyzed cross-dehydrogenation coupling (CDC) involving ether α-C(sp3)-H to construct C-C bonds
Abstract
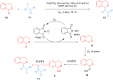
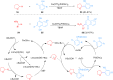
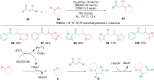
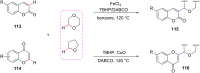
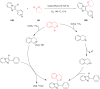
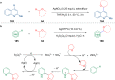
Ether derivatives are widespread as essential building blocks in various drugs, natural products, agrochemicals, and materials. Modern economy requires developing green strategies with improved efficiency and reduction of waste. Due to its atom and step-economy, the cross-dehydrogenative coupling (CDC) reaction has become a major strategy for ether functionalization. This review covers C-H/C-H cross-coupling reactions of ether derivatives with various C-H bond substrates via non-noble metal catalysts (Fe, Cu, Co, Mn, Ni, Zn, Y, Sc, In, Ag). We discuss advances achieved in these CDC reactions and hope to attract interest in developing novel methodologies in this field of organic chemistry.
Keywords: alkylation; cross-dehydrogenation coupling; ether; non-noble metals.
Copyright © 2023, Yu and Xu.
Figures







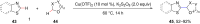









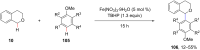



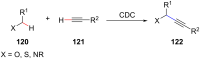





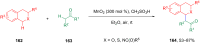



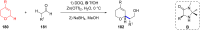




Similar articles
-
Photocatalytic Activation of Less Reactive Bonds and Their Functionalization via Hydrogen-Evolution Cross-Couplings.Acc Chem Res. 2018 Oct 16;51(10):2512-2523. doi: 10.1021/acs.accounts.8b00267. Epub 2018 Oct 3. Acc Chem Res. 2018. PMID: 30280898
-
Exploration of earth-abundant transition metals (Fe, Co, and Ni) as catalysts in unreactive chemical bond activations.Acc Chem Res. 2015 Mar 17;48(3):886-96. doi: 10.1021/ar500345f. Epub 2015 Feb 13. Acc Chem Res. 2015. PMID: 25679917
-
Iron-Catalyzed Cross-Dehydrogenative Coupling.Molecules. 2025 Jan 10;30(2):250. doi: 10.3390/molecules30020250. Molecules. 2025. PMID: 39860120 Free PMC article. Review.
-
Metal-Free Cross-Dehydrogenative Coupling (CDC): Molecular Iodine as a Versatile Catalyst/Reagent for CDC Reactions.Chem Asian J. 2019 Jan 4;14(1):6-30. doi: 10.1002/asia.201801237. Epub 2018 Nov 2. Chem Asian J. 2019. PMID: 30259704 Review.
-
Upgrading Cross-Coupling Reactions for Biaryl Syntheses.Acc Chem Res. 2019 Jan 15;52(1):161-169. doi: 10.1021/acs.accounts.8b00408. Epub 2018 Oct 30. Acc Chem Res. 2019. PMID: 30376296
Cited by
-
Visible-Light Photocatalysis for Sustainable Chromene Synthesis and Functionalization.Chemistry. 2025 May 22;31(29):e202500283. doi: 10.1002/chem.202500283. Epub 2025 Apr 3. Chemistry. 2025. PMID: 40100639 Free PMC article. Review.
-
Catalytic asymmetric carbenoid α-C-H insertion of ether.RSC Adv. 2024 May 13;14(21):15167-15177. doi: 10.1039/d4ra02206h. eCollection 2024 May 2. RSC Adv. 2024. PMID: 38741618 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
