Development of hepatocellular adenomas in a patient with glycogen storage disease Ia treated with growth hormone therapy
- PMID: 37701330
- PMCID: PMC10494510
- DOI: 10.1002/jmd2.12381
Development of hepatocellular adenomas in a patient with glycogen storage disease Ia treated with growth hormone therapy
Abstract
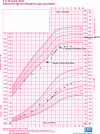
Glycogen storage disease Ia (GSD Ia), also known as von Gierke disease, is caused by pathogenic variants in the G6PC1 gene (OMIM 232200) which encodes glucose-6-phosphatase. Deficiency of glucose-6-phosphatase impairs the processes of gluconeogenesis and glycogenolysis by preventing conversion of glucose-6-phosphate to glucose. Clinical features include fasting hypoglycemia, lactic acidosis, hypertriglyceridemia, hyperuricemia, hepatomegaly, and development of hepatocellular adenomas (HCAs) with potential for malignant transformation. Additionally, patients with GSD Ia often exhibit short stature, in some instances due to growth hormone (GH) deficiency. Patients with short stature caused by GH deficiency typically receive GH injections. Here, we review the literature and describe a female with GSD Ia who had short stature, failure of growth progression, and suspected GH deficiency. This patient received GH injections from ages 11 to 14 years under careful monitoring of an endocrinologist and developed HCAs during that time. To date, there is no reported long-term follow up data on patients with GSD Ia who have received GH therapy, and therefore the clinical outcomes post-GH therapy are unclear.
Keywords: glycogen storage disease; glycogen storage disease Ia; growth hormone therapy; hepatocellular adenoma; von Gierke disease.
© 2023 The Authors. JIMD Reports published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A Liver-Specific Thyromimetic, VK2809, Decreases Hepatosteatosis in Glycogen Storage Disease Type Ia.Thyroid. 2019 Aug;29(8):1158-1167. doi: 10.1089/thy.2019.0007. Thyroid. 2019. PMID: 31337282 Free PMC article.
-
Emerging roles of autophagy in hepatic tumorigenesis and therapeutic strategies in glycogen storage disease type Ia: A review.J Inherit Metab Dis. 2021 Jan;44(1):118-128. doi: 10.1002/jimd.12267. Epub 2020 Jul 2. J Inherit Metab Dis. 2021. PMID: 32474930 Review.
-
Glycogen Storage Disease Type I.2025 Apr 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Apr 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30480935 Free Books & Documents.
-
A patient with glycogen storage disease type Ia combined with chronic hepatitis B infection: a case report.BMC Med Genet. 2019 May 20;20(1):85. doi: 10.1186/s12881-019-0816-9. BMC Med Genet. 2019. PMID: 31109299 Free PMC article.
-
Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex.Curr Mol Med. 2002 Mar;2(2):121-43. doi: 10.2174/1566524024605798. Curr Mol Med. 2002. PMID: 11949931 Review.
Cited by
-
Efficacy of magnetic resonance imaging in managing glycogen storage disease.Orphanet J Rare Dis. 2025 Mar 27;20(1):144. doi: 10.1186/s13023-025-03605-7. Orphanet J Rare Dis. 2025. PMID: 40148892 Free PMC article.
-
Endocrine involvement in hepatic glycogen storage diseases: pathophysiology and implications for care.Rev Endocr Metab Disord. 2024 Aug;25(4):707-725. doi: 10.1007/s11154-024-09880-2. Epub 2024 Apr 1. Rev Endocr Metab Disord. 2024. PMID: 38556561 Free PMC article. Review.
References
-
- Kishnani PS, Austin SL, Abdenur JE, et al. Diagnosis and management of glycogen storage disease type I: a practice guideline of the American College of Medical Genetics and Genomics. Genet Med. 2014;16(11):e1‐e29. - PubMed
-
- Pandey G, Shankar K, Makhija E, et al. Reduced insulin receptor expression enhances proximal tubule gluconeogenesis. J Cell Biochem. 2017;118(2):276‐285. - PubMed
-
- Sechi A, Deroma L, Lapolla A, et al. Fertility and pregnancy in women affected by glycogen storage disease type I, results of a multicenter Italian study. J Inherit Metab Dis. 2013;36(1):83‐89. - PubMed
-
- Rake J, Visser G, Labrune P, Leonard JV, Ullrich K, Smit GPA. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European study on glycogen storage disease type I (ESGSD I). Eur J Pediatr. 2002;161:S20‐S34. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

