On-treatment risks of cirrhosis and hepatocellular carcinoma among a large cohort of predominantly non-Asian patients with non-cirrhotic chronic hepatitis B
- PMID: 37701335
- PMCID: PMC10494462
- DOI: 10.1016/j.jhepr.2023.100852
On-treatment risks of cirrhosis and hepatocellular carcinoma among a large cohort of predominantly non-Asian patients with non-cirrhotic chronic hepatitis B
Abstract
Background & aims: The vast majority of studies evaluating differences in on-treatment risks of hepatocellular carcinoma (HCC) in patients with chronic hepatitis B (CHB) have been conducted in Asia. Data on the course of CHB on antiviral therapy among predominantly non-Asian populations is less well described. We aimed to evaluate overall risks of cirrhosis and HCC and the influence of baseline factors on this risk among a predominantly non-Asian cohort of patients with CHB in the US.
Methods: Using longitudinal data from the national Veterans Affairs database, we evaluated the incidence of cirrhosis or HCC among adults with non-cirrhotic CHB on continuous antiviral therapy. Cumulative incidence functions and adjusted Cox proportional hazards models employed competing risks methods and evaluated overall risk and predictors of developing cirrhosis or HCC while on treatment.
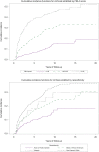
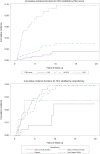
Results: Among 2,496 patients with non-cirrhotic CHB (39.1% African American, 38.4% non-Hispanic White, 18.8% Asian, mean age 58.0 ± 13.4 years), the overall incidences of cirrhosis and HCC were 3.99 per 100 person-years (95% CI 3.66-4.35) and 0.43 per 100 person-years (95% CI 0.33-0.54), respectively. The highest incidences of cirrhosis and HCC were observed in non-Hispanic White patients (5.74 and 0.52 per 100 person-years, respectively), which were significantly higher than in Asian patients (1.93 and 0.17 per 100 person-years, respectively, p <0.0001). On multivariate regression, only baseline FIB-4 score was consistently associated with long-term risk of cirrhosis or HCC.
Conclusions: Using a longitudinal cohort of predominantly non-Asian Veterans with non-cirrhotic CHB on antiviral therapy (an understudied population), we provide important epidemiological data to describe long-term risks of cirrhosis and HCC.
Impact and implications: In one of the largest studies to date of a predominantly non-Asian cohort of patients with non-cirrhotic chronic hepatitis B, we provide important epidemiological data describing the long-term risks of cirrhosis and hepatocellular carcinoma among patients on antiviral therapies. Among this understudied population, the overall incidence of cirrhosis was 3.99 per 100-person-years (95% CI 3.66-4.35) and of HCC was 0.43 per 100-person-years (95% CI 0.33-0.54). These data also emphasize the importance of continued monitoring and HCC surveillance among CHB patients who are maintained on antiviral therapies.
Keywords: HBV; antivirals; cirrhosis; hepatocellular carcinoma; veterans.
© 2023 The Author(s).
Conflict of interest statement
ZY, AC, and WZ report no disclosures. RGG reports grants received from Gilead Sciences; has served as an advisor or consultant to Abbot, AbbVie, Altimunne, Antios, Arrowhead, Dynavax, Eiger, Eisai, Enyo, Genentech, Genlantis, Gerson Lehrman Group, Gilead Sciences, Helios, HepaTX, HepQuant, Intercept, Janssen, Merck, Pfizer, Topography Health, Venatorx, Prodigy, Fibronostics, Fujifilm/Wako, Perspectum, Quest, Sonic Incytes; has served on the data safety monitoring board for Altimmune, Arrowhead, CymaBay Therapeutics, Durect; has served on the speaker’s bureau for AbbVie, BMS, Eisai, Genentech, Gilead Sciences Inc., Intercept; is a minor stock shareholder of RiboSciences, CoCrystal; has received stock options from Eiger, Genlantis, HepQuant, AngioCrine. RJW has received funding (to his institution) from Gilead Sciences and Exact Sciences, and has served as a consultant for Gilead Sciences. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures
Similar articles
-
Association of Baseline Hepatitis B Virus DNA and On-Treatment Risk of Cirrhosis and Hepatocellular Carcinoma.Gastroenterology Res. 2024 Jun;17(3):109-115. doi: 10.14740/gr1735. Epub 2024 Jun 29. Gastroenterology Res. 2024. PMID: 38993547 Free PMC article.
-
Long-term follow-up of cumulative incidence of hepatocellular carcinoma in hepatitis B virus patients without antiviral therapy.World J Gastroenterol. 2021 Mar 21;27(11):1101-1116. doi: 10.3748/wjg.v27.i11.1101. World J Gastroenterol. 2021. PMID: 33776376 Free PMC article. Clinical Trial.
-
Antiviral Therapy Reduces Risk of Cirrhosis in Noncirrhotic HBV Patients Among 4 Urban Safety-Net Health Systems.Am J Gastroenterol. 2021 Jul 1;116(7):1465-1475. doi: 10.14309/ajg.0000000000001195. Am J Gastroenterol. 2021. PMID: 33661148
-
Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy.J Hepatol. 2015 Apr;62(4):956-67. doi: 10.1016/j.jhep.2015.01.002. Epub 2015 Jan 13. J Hepatol. 2015. PMID: 25595883 Review.
-
Magnitude of and prediction for risk of hepatocellular carcinoma in patients with chronic hepatitis B taking entecavir or tenofovir therapy: A systematic review.J Gastroenterol Hepatol. 2020 Oct;35(10):1684-1693. doi: 10.1111/jgh.15078. Epub 2020 May 17. J Gastroenterol Hepatol. 2020. PMID: 32343431
Cited by
-
No Differences in Risk of Cirrhosis or Hepatocellular Carcinoma Among Treatment Naïve Chronic Hepatitis B Patients by Baseline Hepatitis B Viral Load: A Propensity Score Weighted Analysis.J Clin Exp Hepatol. 2025 Jul-Aug;15(4):102540. doi: 10.1016/j.jceh.2025.102540. Epub 2025 Mar 3. J Clin Exp Hepatol. 2025. PMID: 40248346
-
Impact of liver graft steatosis on long-term post-transplant hepatic steatosis and fibrosis via magnetic resonance quantification.Front Med (Lausanne). 2025 Jan 17;11:1502055. doi: 10.3389/fmed.2024.1502055. eCollection 2024. Front Med (Lausanne). 2025. PMID: 39895824 Free PMC article.
-
Hepatitis Delta Virus Testing, Prevalence, and Liver-Related Outcomes Among US Veterans With Chronic Hepatitis B.Gastro Hep Adv. 2024 Oct 25;4(3):100575. doi: 10.1016/j.gastha.2024.10.015. eCollection 2025. Gastro Hep Adv. 2024. PMID: 39906477 Free PMC article.
-
Artificial intelligence in liver cancer - new tools for research and patient management.Nat Rev Gastroenterol Hepatol. 2024 Aug;21(8):585-599. doi: 10.1038/s41575-024-00919-y. Epub 2024 Apr 16. Nat Rev Gastroenterol Hepatol. 2024. PMID: 38627537 Review.
References
-
- Polaris Observatory C. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403. - PubMed
-
- Wong R.J., Jain M.K., Therapondos G., Niu B., Kshirsagar O., Thamer M. Low rates of hepatitis B virus treatment among treatment-eligible patients in safety-net health systems. J Clin Gastroenterol. 2022;56:360–368. - PubMed
LinkOut - more resources
Full Text Sources