Therapy initiation and rapid start with Bictegravir/Emtricitabine/Tenofovir Alafenamide in PLWH
- PMID: 37701387
- PMCID: PMC10495053
- DOI: 10.53854/liim-3103-2
Therapy initiation and rapid start with Bictegravir/Emtricitabine/Tenofovir Alafenamide in PLWH
Abstract
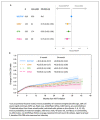
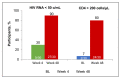
Advanced HIV naive represents an unfavorable prognostic condition due to a persistent high risk of death, increased probability of virological failure, immunologic impairment, clinical progression, and immune reconstitution inflammatory syndrome (IRIS), cumulative adverse drug events, drug-drug interactions and increased healthcare costs. Currently, all international guidelines recommend the rapid initiation of ART, especially in late-stage naive patients. Bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF), due to its efficacy high genetic barrier, good safety profile, and low DDI potential, is one of the regimens recommended for overall rapid initiation by international guidelines. B/F/TAF has been tested in observational or uncontrolled pilot studies (OPERA, RAINBOW), while a large randomized controlled trial is currently ongoing (LAPTOP). In conclusion, B/F/TAF is an ideal combination for the initiation of antiretroviral therapy, particularly in the HIV late presenter or advanced HIV disease patient, even in the context of rapid start or same-day treatment regimens, where the initiation of treatment usually occurs in the absence of information on viral load, CD4 count, biochemical profile and HIV transmitted resistance.
Keywords: Bictegravir; Emtricitabile; PLWH; Tenofovir.
Conflict of interest statement
Conflict of interest None to declare.
Figures

Similar articles
-
Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial.Lancet. 2017 Nov 4;390(10107):2063-2072. doi: 10.1016/S0140-6736(17)32299-7. Epub 2017 Aug 31. Lancet. 2017. PMID: 28867497 Clinical Trial.
-
Comparison of Virological Efficacy of DTG/ABC/3TG and B/F/TAF Regimens and Discontinuation Patterns in Persons Living with Advanced HIV in the Era of Rapid ART: A Retrospective Multicenter Cohort Study.Infect Dis Ther. 2023 Mar;12(3):843-861. doi: 10.1007/s40121-022-00734-5. Epub 2022 Dec 15. Infect Dis Ther. 2023. PMID: 36520332 Free PMC article.
-
Fixed-dose combination bictegravir, emtricitabine, and tenofovir alafenamide in adolescents and children with HIV: week 48 results of a single-arm, open-label, multicentre, phase 2/3 trial.Lancet Child Adolesc Health. 2021 Sep;5(9):642-651. doi: 10.1016/S2352-4642(21)00165-6. Epub 2021 Jul 22. Lancet Child Adolesc Health. 2021. PMID: 34302760 Clinical Trial.
-
The potential role of bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) single-tablet regimen in the expanding spectrum of fixed-dose combination therapy for HIV.HIV Med. 2020 Mar;21 Suppl 1:3-16. doi: 10.1111/hiv.12833. HIV Med. 2020. PMID: 32017355 Review.
-
Bictegravir in a fixed-dose tablet with emtricitabine and tenofovir alafenamide for the treatment of HIV infection: pharmacology and clinical implications.Expert Opin Pharmacother. 2019 Mar;20(4):385-397. doi: 10.1080/14656566.2018.1560423. Epub 2019 Jan 30. Expert Opin Pharmacother. 2019. PMID: 30698467 Review.
References
-
- Late presenters working group in COHERE in Euro-Coord. Mocroft A, Lundgren J, Antinori A, et al. Late presentation for HIV care across Europe: update from the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) study, 2010 to 2013. Euro Surveill . 2015;20(47) doi: 10.2807/1560-7917.ES.2015.20.47.30070. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
