The risk of COVID-19 in IBD patients is increased by urban living and is not influenced by disease activity or intravenous biologics
- PMID: 37701431
- PMCID: PMC10494533
- DOI: 10.3389/fimmu.2023.1243898
The risk of COVID-19 in IBD patients is increased by urban living and is not influenced by disease activity or intravenous biologics
Abstract
Background: Patients with inflammatory bowel disease (IBD) may have a modified immune response to SARS-CoV-2. The objectives were to evaluate the prevalence of COVID-19 in patients treated with infliximab or vedolizumab, to analyze the factors associated with the infection, the impact of treatments and trough levels.
Methods: Patients with IBD treated with intravenous biologics in 14 French centers were included between March and June 2020 and followed-up for 6 months. Blood samples were collected for serologies and trough levels. The analysis of factors associated with COVID-19 was conducted in a matched 1:1 case-control sub-study with positive patients.
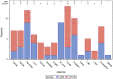
Results: In total, 1026 patients were included (74.9% infliximab). Over the follow-up period, 420 patients reported the occurrence of COVID-19 symptoms; 342 had been tested of whom 18 were positive. At the end of follow-up, 38 patients had a positive serology. Considering both nasal tests and serologies together, 46 patients (4.5%) had been infected. The risk of COVID-19 was related neither to the use of treatments (whatever the trough levels) nor to disease activity. Infections were more frequent when using public transport or living in flats in urban areas.
Conclusions: The prevalence rate of COVID-19 in this IBD population treated with intravenous infliximab or vedolizumab was the same as the one in the French population before the start of the vaccination campaign. The risk was increased by urban living and was not influenced by disease activity or biologics. Sanitary barrier measures remain the best way to protect against SARS-CoV-2 in patients with IBD in biological therapy.
Keywords: Crohn’s disease; SARS-CoV-2; infliximab; trough levels; ulcerative colitis; vedolizumab.
Copyright © 2023 Lelong, Josien, Coste-Burel, Rimbert, Bressollette-Bodin, Nancey, Bouguen, Allez, Serrero, Caillo, Rouillon, Blanc, Laharie, Olivier, Peyrin-Biroulet, Dib, De Maissin, Montuclard, Trang-Poisson, Vavasseur, Gallot, Berthome, Braudeau, Chevreuil, Bourreille and Le Berre.
Conflict of interest statement
SN declares counseling, boards, transports or fees from Abbvie, Biogen, HAC-pharma, Janssen, MSD, Novartis, Pfizer, Takeda, Tillots, BMS, Amgen, Galapagos and Fresenius. Prof. Bouguen received lecture fees from Abbvie, Ferring, MSD, Takeda and Pfizer and consultant fees from Takeda, Janssen. MA reports consulting or lecture fees from AbbVie, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Ferring, Genentech, Gilead, IQVIA, Janssen, Novartis, Pfizer, Roche, Takeda, and Tillots; and grant support from Innate Pharma, Janssen, Takeda, and Genentech/Roche. MS has received lecture or consulting fees from Abbvie, Ferring, Amgen, Celltrion, Janssen, Ferring, Takeda and Tillotts. LC received board and lecture fees from Abbvie, Janssen, Pfizer, Takeda, Amgen. CR has received payment for lectures from Abbvie, Biogen and Galapagos. PB has received payment for lectures from Abbvie, Pfizer, Tillotts, Fresenius Kabi and Gilead. DL declares counseling, boards, transports or fees from Abbvie, Biogaran, Biogen, Ferring, Galapagos, Janssen, Lilly, MSD, Novartis, Pfizer, Prometheus, Roche, Takeda. LP-B has served as a consultant for Abbvie, Alimentiv, Alma Bio Therapeutics, Amgen, Applied Molecular Transport, Arena, Biogen, BMS, Celltrion, CONNECT Biopharm, Cytoki Pharma, Enthera, Ferring, Fresenius Kabi, Galapagos, Genentech, Gilead, Gossamer Bio, GSK, HAC-Pharma, IAG Image Analysis, Index Pharmaceuticals, Inotrem, Janssen, Lilly, Medac, Mopac, Morphic, MSD, Norgine, Novartis, OM Pharma, ONO Pharma, OSE Immunotherapeutics, Pandion Therapeutics, Par’Immune, Pfizer, Prometheus, Protagonist, Roche, Sandoz, Takeda, Theravance, Thermo Fisher, Tigenix, Tillots, Viatris, Vifor, Ysopia, Abivax; has received payment for lectures from Galapagos, AbbVie, Janssen, Genentech, Ferring, Tillots, Celltrion, Takeda, Pfizer, Sandoz, Biogen, MSD, Amgen, Vifor, Arena, Lilly, Gilead, Viatris, Medac, Sanofi; reports grant support from Takeda, Fresenius Kabi, Celltrion; has received meeting support fees from Galapagos, AbbVie, Janssen, Genentech, Ferring, Tillots, Celltrion, Takeda, Pfizer, Gossamer Bio, Sandoz, MSD, Amgen, Lilly, Gilead, Thermo Fisher, Medac, CONNECT Biopharm. AM has received payment for lectures from Galapagos. CT-P has reveived lecture fees from Ferring, Janssen, Mayoly spindler, Norgine, Abbvie, MSD, and Takeda. AB declares lecture or consulting fees from Abbvie, Amgen, Celltrion, Ferring, Fresenius Kabi, Galapagos, Gilead, Janssen, MSD, OSE Immunotherapeutics, Pfizer, Roche, Takeda, and Tillotts. CLB has served as a consultant for Abbvie, Janssen and Gilead; has received payment for lectures from Abbvie, Amgen, Celltrion, Ferring, Fresenius Kabi, Galapagos, Janssen, Lilly, MSD, Pfizer, and Takeda; reports grant support from Abbvie and Takeda; has received meeting support fees from Abbvie, Ferring, Fresenius Kabi, Galapagos, Janssen, Lilly, Pfizer, Sandoz, and Takeda. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- GBD 2017 Inflammatory Bowel Disease Collaborators . The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol (2020) 5:17–30. doi: 10.1016/S2468-1253(19)30333-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous