Mechanism and clinical application of thymosin in the treatment of lung cancer
- PMID: 37701432
- PMCID: PMC10493777
- DOI: 10.3389/fimmu.2023.1237978
Mechanism and clinical application of thymosin in the treatment of lung cancer
Abstract
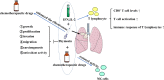
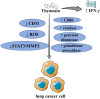
Cancer is one of the leading causes of death worldwide. The burden of cancer on public health is becoming more widely acknowledged. Lung cancer has one of the highest incidence and mortality rates of all cancers. The prevalence of early screening, the emergence of targeted therapy, and the development of immunotherapy have all significantly improved the overall prognosis of lung cancer patients. The current state of affairs, however, is not encouraging, and there are issues like poor treatment outcomes for some patients and extremely poor prognoses for those with advanced lung cancer. Because of their potent immunomodulatory capabilities, thymosin drugs are frequently used in the treatment of tumors. The effectiveness of thymosin drugs in the treatment of lung cancer has been demonstrated in numerous studies, which amply demonstrates the potential and future of thymosin drugs for the treatment of lung cancer. The clinical research on thymosin peptide drugs in lung cancer and the basic research on the mechanism of thymosin drugs in anti-lung cancer are both systematically summarized and analyzed in this paper, along with future research directions.
Keywords: immunotherapy; lung cancer; therapy; thymalfasin; thymopentin; thymosin; thymosin alpha 1.
Copyright © 2023 Liu and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Thymosin alpha 1 in the treatment of cancer: from basic research to clinical application.Int J Immunopharmacol. 2000 Dec;22(12):1067-76. doi: 10.1016/s0192-0561(00)00075-8. Int J Immunopharmacol. 2000. PMID: 11137613 Review.
-
Prospective evaluation of thymosin fraction V immunotherapy in patients with non-small cell lung cancer receiving vindesine, doxorubicin, and cisplatin (VAP) chemotherapy.Am J Clin Oncol. 1984 Oct;7(5):399-404. doi: 10.1097/00000421-198410000-00002. Am J Clin Oncol. 1984. PMID: 6391140 Clinical Trial.
-
Thymosin alpha 1 - Reimagine its broader applications in the immuno-oncology era.Int Immunopharmacol. 2023 Apr;117:109952. doi: 10.1016/j.intimp.2023.109952. Epub 2023 Mar 3. Int Immunopharmacol. 2023. PMID: 36871535
-
Effects of thymosin in vitro in cancer patients and correlation with clinical course after thymosin immunotherapy.Ann N Y Acad Sci. 1979;332:135-47. doi: 10.1111/j.1749-6632.1979.tb47107.x. Ann N Y Acad Sci. 1979. PMID: 316979 No abstract available.
-
Thymosin α1 in melanoma: from the clinical trial setting to the daily practice and beyond.Ann N Y Acad Sci. 2012 Oct;1270:8-12. doi: 10.1111/j.1749-6632.2012.06757.x. Ann N Y Acad Sci. 2012. PMID: 23050811 Review.
Cited by
-
Evidence based on Mendelian randomization: Causal relationship between mitochondrial biological function and lung cancer and its subtypes.Neoplasia. 2023 Dec;46:100950. doi: 10.1016/j.neo.2023.100950. Epub 2023 Nov 16. Neoplasia. 2023. PMID: 37976568 Free PMC article.
-
Phenotypic drug discovery: a case for thymosin alpha-1.Front Med (Lausanne). 2024 Jun 6;11:1388959. doi: 10.3389/fmed.2024.1388959. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38903817 Free PMC article. Review.
-
Identifying Lipid Metabolism-Related Therapeutic Targets and Diagnostic Markers for Lung Adenocarcinoma by Mendelian Randomization and Machine Learning Analysis.Thorac Cancer. 2025 Mar;16(6):e70020. doi: 10.1111/1759-7714.70020. Thorac Cancer. 2025. PMID: 40107973 Free PMC article.
-
A preliminary analysis of integrating thymosin α1 into concurrent chemoradiotherapy and consolidative immunotherapy in unresectable locally advanced non-small cell lung cancer.Transl Lung Cancer Res. 2025 Jul 31;14(7):2710-2722. doi: 10.21037/tlcr-2025-190. Epub 2025 Jul 28. Transl Lung Cancer Res. 2025. PMID: 40799433 Free PMC article.
References
-
- Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, Harvey JD, et al. . Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: A systematic analysis for the global burden of disease study 2019. JAMA Oncol (2022) 8(3):420–44. doi: 10.1001/jamaoncol.2021.6987 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

