Enhanced cell viscosity: A new phenotype associated with lamin A/C alterations
- PMID: 37701573
- PMCID: PMC10494210
- DOI: 10.1016/j.isci.2023.107714
Enhanced cell viscosity: A new phenotype associated with lamin A/C alterations
Abstract
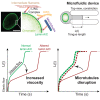
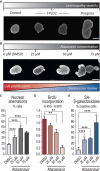
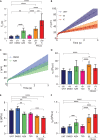
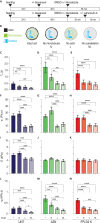
Lamin A/C is a well-established key contributor to nuclear stiffness and its role in nucleus mechanical properties has been extensively studied. However, its impact on whole-cell mechanics has been poorly addressed, particularly concerning measurable physical parameters. In this study, we combined microfluidic experiments with theoretical analyses to quantitatively estimate the whole-cell mechanical properties. This allowed us to characterize the mechanical changes induced in cells by lamin A/C alterations and prelamin A accumulation resulting from atazanavir treatment or lipodystrophy-associated LMNA R482W pathogenic variant. Our results reveal a distinctive increase in long-time viscosity as a signature of cells affected by lamin A/C alterations. Furthermore, they show that the whole-cell response to mechanical stress is driven not only by the nucleus but also by the nucleo-cytoskeleton links and the microtubule network. The enhanced cell viscosity assessed with our microfluidic assay could serve as a valuable diagnosis marker for lamin-related diseases.
Keywords: Biological sciences; Biotechnology; Cell biology.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Lipodystrophy-linked LMNA p.R482W mutation induces clinical early atherosclerosis and in vitro endothelial dysfunction.Arterioscler Thromb Vasc Biol. 2013 Sep;33(9):2162-71. doi: 10.1161/ATVBAHA.113.301933. Epub 2013 Jul 11. Arterioscler Thromb Vasc Biol. 2013. PMID: 23846499
-
LMNA missense mutations causing familial partial lipodystrophy do not lead to an accumulation of prelamin A.Nucleus. 2016 Sep 2;7(5):512-521. doi: 10.1080/19491034.2016.1242542. Nucleus. 2016. PMID: 27841971 Free PMC article.
-
Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration.Biophys J. 2007 Oct 1;93(7):2542-52. doi: 10.1529/biophysj.106.102426. Epub 2007 Jul 13. Biophys J. 2007. PMID: 17631533 Free PMC article.
-
Molecular and Mechanobiological Pathways Related to the Physiopathology of FPLD2.Cells. 2020 Aug 23;9(9):1947. doi: 10.3390/cells9091947. Cells. 2020. PMID: 32842478 Free PMC article. Review.
-
Lamin A involvement in ageing processes.Ageing Res Rev. 2020 Sep;62:101073. doi: 10.1016/j.arr.2020.101073. Epub 2020 May 21. Ageing Res Rev. 2020. PMID: 32446955 Review.
Cited by
-
Deformation under flow and morphological recovery of cancer cells.Lab Chip. 2024 Aug 6;24(16):3930-3944. doi: 10.1039/d4lc00246f. Lab Chip. 2024. PMID: 38993177 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

