Regional differences in islet amyloid deposition in the residual pancreas with new-onset diabetes secondary to pancreatic ductal adenocarcinoma
- PMID: 37701698
- PMCID: PMC10494581
- DOI: 10.4240/wjgs.v15.i8.1703
Regional differences in islet amyloid deposition in the residual pancreas with new-onset diabetes secondary to pancreatic ductal adenocarcinoma
Abstract
Background: Islet amyloid deposition and reduced β-cell mass are pathological hallmarks in type 2 diabetes mellitus subjects. To date, the pathological features of the islets in diabetes secondary to pancreatic ductal adenocarcinoma (PDAC) have not been specifically addressed.
Aim: To provide further insight into the relationship between islet amyloid deposition of the residual pancreas in PDAC patients and to explore whether regional differences (proximal vs distal residual pancreas) are associated with islet amyloid deposition.
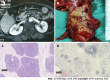
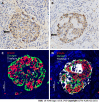
Methods: We retrospectively collected clinical information and pancreatic tissue removed from tumors of 45 PDAC patients, including 14 patients with normal glucose tolerance (NGT), 16 patients with prediabetes and 15 new-onset diabetes (NOD) patients diagnosed before surgery by an oral glucose tolerance test at West China Hospital from July 2017 to June 2020. Pancreatic volume was calculated by multiplying the estimated area of pancreatic tissue on each image slice by the interval between slices based on abdominal computer tomography scans. Several sections of paraffin-embedded pancreas specimens from both the proximal and/or distal regions remote from the tumor were stained as follows: (1) Hematoxylin and eosin for general histological appearance; (2) hematoxylin and insulin for the determination of fractional β-cell area (immunohistochemistry); and (3) quadruple insulin, glucagon, thioflavin T and DAPI staining for the determination of β-cell area, α-cell area and amyloid deposits.
Results: Screening for pancreatic histologic features revealed that duct obstruction with islet amyloid deposition, fibrosis and marked acinar atrophy were robust in the distal pancreatic regions but much less robust in the proximal regions, especially in the prediabetes and NOD groups. Consistent with this finding, the remnant pancreatic volume was markedly decreased in the NOD group by nearly one-half compared with that in the NGT group (37.35 ± 12.16 cm3 vs 69.79 ± 18.17 cm3, P < 0.001). As expected, islets that stained positive for amyloid (islet amyloid density) were found in the majority of PDAC cases. The proportion of amyloid/islet area (severity of amyloid deposition) was significantly higher in both prediabetes and NOD patients than in NGT patients (P = 0.002; P < 0.0001, respectively). We further examined the regional differences in islet amyloid deposits. Islet amyloid deposit density was robustly increased by approximately 8-fold in the distal regions compared with that in the proximal regions in the prediabetes and NOD groups (3.98% ± 3.39% vs 0.50% ± 0.72%, P = 0.01; 12.03% vs 1.51%, P = 0.001, respectively).
Conclusion: In conclusion, these findings suggest that robust alterations of the distal pancreas due to tumors can disturb islet function and structure with islet amyloid formation, which may be associated with the pathogenesis of NOD secondary to PDAC.
Keywords: Amyloid deposits; Diabetes; Islet amyloid polypeptide; Pancreatic ductal adenocarcinoma; Residual pancreas.
©The Author(s) 2023. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All authors report no relevant conflict of interest for this article.
Figures
Similar articles
-
Remnant Pancreas Volume Affects New-Onset Impaired Glucose Homeostasis Secondary to Pancreatic Cancer.Biomedicines. 2024 Jul 24;12(8):1653. doi: 10.3390/biomedicines12081653. Biomedicines. 2024. PMID: 39200119 Free PMC article.
-
Pancreas Atrophy and Islet Amyloid Deposition in Patients With Elderly-Onset Type 2 Diabetes.J Clin Endocrinol Metab. 2017 Sep 1;102(9):3162-3171. doi: 10.1210/jc.2016-3735. J Clin Endocrinol Metab. 2017. PMID: 28505316
-
Islet amyloid polypeptide in pancreatic islets from type 2 diabetic subjects.Islets. 2012 May-Jun;4(3):223-32. doi: 10.4161/isl.20477. Islets. 2012. PMID: 22847497 Free PMC article.
-
Pancreatic pathology in non-insulin dependent diabetes (NIDDM).Diabetes Res Clin Pract. 1995 Aug;28 Suppl:S39-47. doi: 10.1016/0168-8227(95)01075-o. Diabetes Res Clin Pract. 1995. PMID: 8529518 Review.
-
Pathogenesis of feline diabetes mellitus.Vet Clin North Am Small Anim Pract. 1995 May;25(3):527-52. doi: 10.1016/s0195-5616(95)50051-8. Vet Clin North Am Small Anim Pract. 1995. PMID: 7660530 Review.
Cited by
-
Can AI Be Useful in the Early Detection of Pancreatic Cancer in Patients with New-Onset Diabetes?Biomedicines. 2025 Mar 31;13(4):836. doi: 10.3390/biomedicines13040836. Biomedicines. 2025. PMID: 40299428 Free PMC article. Review.
-
The Silent Killer: An Autopsy Case of Basilar Artery Thrombosis With Diabetes Mellitus, Metabolic Dysfunction-Associated Steatotic Liver Disease, and Gastroesophageal Reflux Disease.Cureus. 2025 May 8;17(5):e83701. doi: 10.7759/cureus.83701. eCollection 2025 May. Cureus. 2025. PMID: 40486373 Free PMC article.
References
-
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81–S90. - PubMed
-
- Cui Y, Andersen DK. Pancreatogenic diabetes: special considerations for management. Pancreatology. 2011;11:279–294. - PubMed
-
- Hart PA, Bellin MD, Andersen DK, Bradley D, Cruz-Monserrate Z, Forsmark CE, Goodarzi MO, Habtezion A, Korc M, Kudva YC, Pandol SJ, Yadav D, Chari ST Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer(CPDPC) Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol. 2016;1:226–237. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

