Efficacy, safety, and correlative biomarkers of bintrafusp alfa in recurrent or metastatic nasopharyngeal cancer patients: a phase II clinical trial
- PMID: 37701718
- PMCID: PMC10493598
- DOI: 10.1016/j.lanwpc.2023.100898
Efficacy, safety, and correlative biomarkers of bintrafusp alfa in recurrent or metastatic nasopharyngeal cancer patients: a phase II clinical trial
Abstract
Background: The strategy of dual blockade of TGF-β and PD-L1 pathways has not been previously tested in platinum-refractory recurrent or metastatic nasopharyngeal cancer (R/M NPC) patients. This study aimed to evaluate the safety and efficacy of bintrafusp alfa in refractory R/M NPC patients.
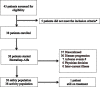
Methods: In this single-arm, single-centre phase II clinical trial, 38 histologically confirmed R/M NPC patients were enrolled and administered with bintrafusp alfa every 2 weeks. Primary endpoint was objective response rate (ORR) per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1). Secondary endpoints included progression-free survival (PFS), overall survival (OS), duration of response (DOR), and safety.
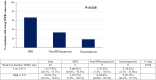
Findings: Thirty-eight patients were accrued (33 men; median age, 54 years). ORR was 23.7% (complete response, n = 2; partial response, n = 7). The median DOR was 19.2 months, median PFS was 2.3 months, median OS was 17.0 months, and 1-year OS rate was 63.2%. Unfortunately, 25 patients (65.7%) progressed within 8 weeks of treatment, 15 patients (39.5%) and 8 patients (21.1%) developed hyper-progressive disease (HPD) per RECIST v1.1 and tumor growth rate (TGR) ratio respectively. Sixteen patients (42.4%) experienced ≥ grade 3 treatment-related adverse events (TRAEs), most commonly anemia (n = 9, 23.7%) and secondary malignancies (n = 4, 10.5%). TRAEs led to permanent treatment discontinuation in 7 patients. Patients with strong suppression of plasma TGFβ1 level at week 8 were unexpectedly associated with worse ORR (9.1% vs 44.4%, P = 0.046) and development of HPD. There was no correlation between PD-L1 expression and ORR.
Interpretation: Bintrafusp alfa demonstrated modest activity in R/M NPC but high rates of HPD and treatment discontinuation secondary to TRAEs are concerning.
Funding: The project was supported by Alice Ho Miu Ling Nethersole Charity Foundation Professorship Endowed Fund and Merck KGaA.
Keywords: Bintrafusp alfa; Correlative biomarkers; Efficacy; Recurrent or metastatic nasopharyngeal cancer; Safety.
© 2023 The Author(s).
Conflict of interest statement
All the authors declare no potential conflicts of interest.
Figures

References
-
- Carioli G., Negri E., Kawakita D., Garavello W., La Vecchia C., Malvezzi M. Global trends in nasopharyngeal cancer mortality since 1970 and predictions for 2020: focus on low-risk areas. Int J Cancer. 2017;140:2256–2264. - PubMed
-
- Chua M.L.K., Wee J.T.S., Hui E.P., Chan A.T.C. Nasopharyngeal carcinoma. Lancet. 2016;387:1012–1024. - PubMed
-
- Lee A.W., Ma B.B., Ng W.T., Chan A.T. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33:3356–3364. - PubMed
-
- Zhang L., Huang Y., Hong S., et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388:1883–1892. - PubMed
-
- Ma B.B., Chan A.T. Recent perspectives in the role of chemotherapy in the management of advanced nasopharyngeal carcinoma. Cancer. 2005;103:22–31. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

