Enhanced Osteogenic Activity and Bone Repair Ability of PLGA/MBG Scaffolds Doped with ZIF-8 Nanoparticles Loaded with BMP-2
- PMID: 37701821
- PMCID: PMC10493152
- DOI: 10.2147/IJN.S423985
Enhanced Osteogenic Activity and Bone Repair Ability of PLGA/MBG Scaffolds Doped with ZIF-8 Nanoparticles Loaded with BMP-2
Abstract
Background: Tissue engineering scaffolds are porous and can be loaded with growth factors to promote osteogenesis and bone repair, which can solve the problem of clinical bone defects. The direct loading of growth factors on scaffolds is hindered by the disadvantages of low loading capacities, and uncontrollable burst release. Zeolitic imidazolate framework-8 (ZIF-8) has osteoinductive activity and drug-loading potential and can be loaded with growth factors to achieve sustained release. In this study, we aimed to establish a sustained release system of composite scaffolds loaded with growth factors to achieve the goal of slow controlled release and effective bone repair.
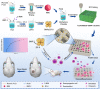
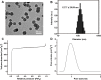
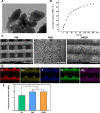
Methods: ZIF‑8 nanoparticles loaded with bone morphogenetic protein-2 (BMP-2) were incorporated into poly-(lactide-co-glycolide)/mesoporous bioactive glass (PLGA/MBG) porous scaffolds by a 3D-printing method. The surface morphology, chemical properties and BMP-2 release of the prepared scaffold were investigated. The osteoblast adhesion, proliferation, spreading, and osteogenic differentiation in vitro and the bone repair ability in vivo of the PLGA/MBG/ZIF-8/BMP-2 (PMZB) scaffold were evaluated, and compared with those of PLGA/MBG (PM) and PLGA/MBG/ZIF-8 (PMZ) scaffolds.
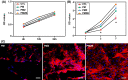
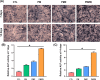
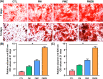
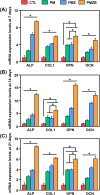
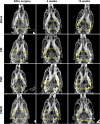
Results: The results showed that the PMZB scaffold exhibited a slow and continuous BMP-2 release pattern, enhanced osteoblast adhesion, proliferation, spreading and osteogenic differentiation in vitro, and promoted new bone formation and bone repair in vivo.
Conclusion: The PLGA/MBG/ZIF-8/BMP-2 porous scaffold could continuously and slowly release BMP-2, enhance osteogenic activity, and promote new bone formation and bone repair at bone defects. The PMZB scaffold can be used as a bone graft material to repair bone defect at non-weight-bearing sites.
Keywords: bone repair; controlled release; mesoporous bioactive glass; scaffold; zeolitic imidazolate frameworks-8.
© 2023 Ma et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Mesoporous bioactive glass surface modified poly(lactic-co-glycolic acid) electrospun fibrous scaffold for bone regeneration.Int J Nanomedicine. 2015 Jun 2;10:3815-27. doi: 10.2147/IJN.S82543. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26082632 Free PMC article.
-
Supercritical CO2 foamed composite scaffolds incorporating bioactive lipids promote vascularized bone regeneration via Hif-1α upregulation and enhanced type H vessel formation.Acta Biomater. 2019 Aug;94:253-267. doi: 10.1016/j.actbio.2019.05.066. Epub 2019 May 31. Acta Biomater. 2019. PMID: 31154054
-
A novel vehicle-like drug delivery 3D printing scaffold and its applications for a rat femoral bone repairing in vitro and in vivo.Int J Biol Sci. 2020 Apr 1;16(11):1821-1832. doi: 10.7150/ijbs.37552. eCollection 2020. Int J Biol Sci. 2020. PMID: 32398952 Free PMC article.
-
Investigations into the effects of scaffold microstructure on slow-release system with bioactive factors for bone repair.Front Bioeng Biotechnol. 2023 Sep 14;11:1230682. doi: 10.3389/fbioe.2023.1230682. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37781533 Free PMC article. Review.
-
Therapeutic Potential of Nano-Sustained-Release Factors for Bone Scaffolds.J Funct Biomater. 2025 Apr 9;16(4):136. doi: 10.3390/jfb16040136. J Funct Biomater. 2025. PMID: 40278244 Free PMC article. Review.
Cited by
-
Folate-Targeted Nanocarriers Co-Deliver Ganciclovir and miR-34a-5p for Combined Anti-KSHV Therapy.Int J Mol Sci. 2024 Mar 2;25(5):2932. doi: 10.3390/ijms25052932. Int J Mol Sci. 2024. PMID: 38474177 Free PMC article.
-
Magnetic Graphene Oxide Nanocomposites Boosts Craniomaxillofacial Bone Regeneration by Modulating circAars/miR-128-3p/SMAD5 Signaling Axis.Int J Nanomedicine. 2024 Apr 3;19:3143-3166. doi: 10.2147/IJN.S454718. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38585472 Free PMC article.
-
Metal-organic framework (MOF)-bioactive glass (BG) systems for biomedical applications - A review.Mater Today Bio. 2024 Dec 18;30:101413. doi: 10.1016/j.mtbio.2024.101413. eCollection 2025 Feb. Mater Today Bio. 2024. PMID: 39834480 Free PMC article. Review.
-
Anti-Infection Efficacy, Osteogenesis Potential, and Biocompatibility of 3D Printed PLGA/Nano-Hydroxyapatite Porous Scaffolds Grafted with Vancomycin/DOPA/rhBMP-2 in Infected Rabbit Bone Defects.Int J Nanomedicine. 2025 May 21;20:6399-6421. doi: 10.2147/IJN.S514978. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40416730 Free PMC article.
-
3D printed scaffolds loaded with BMP-2 for bone defect regeneration: a systematic review and meta-analysis.Front Physiol. 2025 Jul 30;16:1641937. doi: 10.3389/fphys.2025.1641937. eCollection 2025. Front Physiol. 2025. PMID: 40809287 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

