Comparative safety of different sodium-glucose transporter 2 inhibitors in patients with type 2 diabetes: a systematic review and network meta-analysis of randomized controlled trials
- PMID: 37701900
- PMCID: PMC10494439
- DOI: 10.3389/fendo.2023.1238399
Comparative safety of different sodium-glucose transporter 2 inhibitors in patients with type 2 diabetes: a systematic review and network meta-analysis of randomized controlled trials
Abstract
Backgrounds: The safety of different sodium-glucose transporter 2 (SGLT-2) inhibitors remains uncertain due to the lack of head-to-head comparisons.
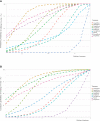
Methods: This network meta-analysis (NMA) was performed to compare the safety of nine SGLT-2 inhibitors in patients with type 2 diabetes (T2DM). PubMed, Embase, Cochrane Central Register of Controlled Trials and ClinicalTrials.gov were searched for studies published in English before August 30, 2022. Published and unpublished randomized controlled trials (RCTs) comparing the safety of individual SGLT-2 inhibitors in patients with T2DM were included. A Bayesian NMA with random effects model was applied. Subgroup and sensitivity analyses were performed. The quality of the evidence was evaluated using the Confidence in Network Meta-Analysis framework.
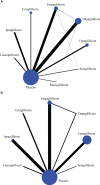
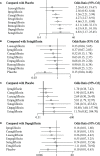
Results: Nine SGLT-2 inhibitors were evaluated in 113 RCTs (12 registries) involving 105,293 adult patients. Reproductive tract infections (RTIs) were reported in 1,967 (4.51%) and 276 (1.01%) patients in the SGLT-2 inhibitor and placebo groups, respectively. Furthermore, pollakiuria was reported in 233 (2.66%) and 45 (0.84%) patients, respectively. Compared to placebo, a significantly higher risk of RTIs was observed with canagliflozin, ertugliflozin, empagliflozin, remogliflozin, dapagliflozin, and sotagliflozin, but not with luseogliflozin and ipragliflozin, regardless of gender. An increased risk of pollakiuria was observed with dapagliflozin [odds ratio (OR) 10.40, 95% confidence interval (CI) 1.60-157.94) and empagliflozin (OR 5.81, 95%CI 1.79-32.97). Remogliflozin (OR 6.45, 95%CI 2.18-27.79) and dapagliflozin (OR 1.33, 95%CI 1.10-1.62) were associated with an increased risk of urinary tract infections (UTIs). Instead, the included SGLT-2 inhibitors had a protective effect against acute kidney injury (AKI). No significant differences were found for hypovolemia, renal impairment or failure, fracture, diabetic ketoacidosis (DKA), amputation, and severe hypoglycemia between the SGLT-2 inhibitor and the placebo groups.
Conclusion: In patients with T2DM, dapagliflozin was associated with an increased risk of RTIs, pollakiuria, and UTIs. Empagliflozin increased the risk of RTIs and pollakiuria. Remogliflozin increased the risk of UTIs. None of the SGLT-2 inhibitors showed a significant difference from the placebo for hypovolemia, renal impairment or failure, fracture, DKA, amputation, and severe hypoglycemia. The findings guide the selection of SGLT-2 inhibitors for patients with T2DM based on the patient's profiles to maximize safety.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier CRD42022334644.
Keywords: hypovolemia; network meta-analysis; pollakiuria; reproductive tract infections; sodium-glucose transporter 2 inhibitors.
Copyright © 2023 Li, Liu, Zhang, Geng, Gu, Wang, Liu, Xie and Liang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Comparative safety of different recommended doses of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis of randomized clinical trials.Front Endocrinol (Lausanne). 2023 Nov 10;14:1256548. doi: 10.3389/fendo.2023.1256548. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38027214 Free PMC article.
-
Comparative cardiovascular benefits of individual SGLT2 inhibitors in type 2 diabetes and heart failure: a systematic review and network meta-analysis of randomized controlled trials.Front Endocrinol (Lausanne). 2023 Dec 20;14:1216160. doi: 10.3389/fendo.2023.1216160. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38179304 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Urinary tract and genital infections in patients with type 2 diabetes treated with sodium-glucose co-transporter 2 inhibitors: A meta-analysis of randomized controlled trials.Diabetes Obes Metab. 2017 Mar;19(3):348-355. doi: 10.1111/dom.12825. Epub 2016 Dec 19. Diabetes Obes Metab. 2017. PMID: 27862830
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
Cited by
-
Rising tide: The global surge of type 2 diabetes in children and adolescents demands action now.World J Diabetes. 2024 May 15;15(5):797-809. doi: 10.4239/wjd.v15.i5.797. World J Diabetes. 2024. PMID: 38766426 Free PMC article.
-
Efficacy and safety of 11 sodium-glucose cotransporter-2 inhibitors at different dosages in type 2 diabetes mellitus patients inadequately controlled with metformin: a Bayesian network meta-analysis.BMJ Open. 2025 Feb 26;15(2):e088687. doi: 10.1136/bmjopen-2024-088687. BMJ Open. 2025. PMID: 40010842 Free PMC article.
-
Clinical Recommendations for Managing Genitourinary Adverse Effects in Patients Treated with SGLT-2 Inhibitors: A Multidisciplinary Expert Consensus.J Clin Med. 2024 Oct 30;13(21):6509. doi: 10.3390/jcm13216509. J Clin Med. 2024. PMID: 39518647 Free PMC article. Review.
-
Serum uric acid reduction through SGLT2 inhibitors: evidence from a systematic review and meta-analysis.Front Pharmacol. 2025 Jun 19;16:1551390. doi: 10.3389/fphar.2025.1551390. eCollection 2025. Front Pharmacol. 2025. PMID: 40612743 Free PMC article.
-
The effect of Empagliflozin on echocardiographic parameters in diabetic patients after acute myocardial infarction: A systematic review and meta-analysis with trial sequential analysis.Ir J Med Sci. 2024 Oct;193(5):2223-2238. doi: 10.1007/s11845-024-03744-z. Epub 2024 Jul 3. Ir J Med Sci. 2024. PMID: 38958683
References
-
- Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. . 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care (2020) 43(2):487–93. doi: 10.2337/dc20-er07 - DOI - PMC - PubMed
-
- Kaku K, Watada H, Iwamoto Y, Utsunomiya K, Terauchi Y, Tobe K, et al. . Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter–2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo–controlled, double–blind, parallel–group comparative study. Cardiovasc Diabetol (2014) 13(1):65. doi: 10.1186/1475-2840-13-65 - DOI - PMC - PubMed
-
- Seino Y, Sasaki T, Fukatsu A, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a 12–week, randomized, placebo–controlled, phase II study. Curr Med Res Opin (2014) 30(7):1219–30. doi: 10.1185/03007995.2014.901943 - DOI - PubMed
-
- Rosenstock J, Frias J, Pall D, Charbonnel B, Pascu R, Saur D, et al. . Effect of ertugliflozin on glucose control, body weight, blood pressure and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET). Diabetes Obes Metab (2018) 20(3):520–9. doi: 10.1111/dom.13103 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

