Genetic and histological relationship between pheromone-secreting tissues of the musk gland and skin of juvenile Chinese forest musk deer (Moschus berezovskii Flerov, 1929)
- PMID: 37701957
- PMCID: PMC10500096
- DOI: 10.1631/jzus.B2200692
Genetic and histological relationship between pheromone-secreting tissues of the musk gland and skin of juvenile Chinese forest musk deer (Moschus berezovskii Flerov, 1929)
Abstract
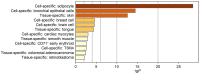
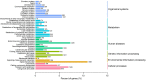
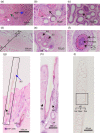
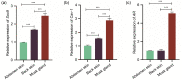
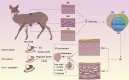
BACKGROUND: The musk glands of adult male Chinese forest musk deer (Moschus berezovskii Flerov, 1929) (FMD), which are considered as special skin glands, secrete a mixture of sebum, lipids, and proteins into the musk pod. Together, these components form musk, which plays an important role in attracting females during the breeding season. However, the relationship between the musk glands and skin of Chinese FMD remains undiscovered. Here, the musk gland and skin of Chinese FMD were examined using histological analysis and RNA sequencing (RNA-seq), and the expression of key regulatory genes was evaluated to determine whether the musk gland is derived from the skin. METHODS: A comparative analysis of musk gland anatomy between juvenile and adult Chinese FMD was conducted. Then, based on the anatomical structure of the musk gland, skin tissues from the abdomen and back as well as musk gland tissues were obtained from three juvenile FMD. These tissues were used for RNA-seq, hematoxylin-eosin (HE) staining, immunohistochemistry (IHC), western blot (WB), and quantitative real-time polymerase chain reaction (qRT-PCR) experiments. RESULTS: Anatomical analysis showed that only adult male FMD had a complete glandular organ and musk pod, while juvenile FMD did not have any well-developed musk pods. Transcriptomic data revealed that 88.24% of genes were co-expressed in the skin and musk gland tissues. Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathway analysis found that the genes co-expressed in the abdomen skin, back skin, and musk gland were enriched in biological development, endocrine system, lipid metabolism, and other pathways. Gene Ontology (GO) enrichment analysis indicated that the genes expressed in these tissues were enriched in biological processes such as multicellular development and cell division. Moreover, the Metascape predictive analysis tool demonstrated that genes expressed in musk glands were skin tissue-specific. qRT-PCR and WB revealed that sex-determining region Y-box protein 9 (Sox9),Caveolin-1 (Cav-1), andandrogen receptor (AR) were expressed in all three tissues, although the expression levels differed among the tissues. According to the IHC results, Sox9 and AR were expressed in the nuclei of sebaceous gland, hair follicle, and musk gland cells, whereas Cav-1 was expressed in the cell membrane. CONCLUSIONS: The musk gland of Chinese FMD may be a derivative of skin tissue, and Sox9, Cav-1, and AR may play significant roles in musk gland development.
中国林麝成年雄性的香腺,被认为是一种特殊的皮肤腺体,能够分泌皮脂、脂质和蛋白质等物质进入林麝的香囊。香囊中的物质成熟后称之为麝香,雄性林麝分泌的麝香用来在繁殖季节吸引雌性林麝,且在药用价值和领域标记方面起着重要作用。由于林麝作为国家一级保护动物,组织样品非常珍贵且难以获得,因此中国林麝的麝香腺与皮肤之间的关系仍未被发现。本研究利用组织形态学、RNA测序(RNA-seq)和免疫组化等方法对中国林麝的麝香腺和皮肤进行了检测,并评估了关键调控基因的表达,同时分析皮肤(背部皮肤和腹部皮肤)和香腺的组织结构以确定麝香腺是否来源于皮肤。本研究主要得到如下结果:(1)林麝皮肤和香腺组织共同表达的基因高达88.24%,且Metascap预测分析工具证明麝香腺体中表达的基因具有皮肤组织特异性;(2)免疫化学和分子生物实验证实关键调控基因在皮肤和香腺中均有表达;(3)组织形态学分析结果证实在组织空间结构上香腺和皮肤组织中均含有皮脂腺,推测其原因是麝香中主要成分是脂肪酸(71.55%),且皮脂腺和毛囊作为一个结构单元参与林麝信息素的分泌。.
中国林麝成年雄性的香腺,被认为是一种特殊的皮肤腺体,能够分泌皮脂、脂质和蛋白质等物质进入林麝的香囊。香囊中的物质成熟后称之为麝香,雄性林麝分泌的麝香用来在繁殖季节吸引雌性林麝,且在药用价值和领域标记方面起着重要作用。由于林麝作为国家一级保护动物,组织样品非常珍贵且难以获得,因此中国林麝的麝香腺与皮肤之间的关系仍未被发现。本研究利用组织形态学、RNA测序(RNA-seq)和免疫组化等方法对中国林麝的麝香腺和皮肤进行了检测,并评估了关键调控基因的表达,同时分析皮肤(背部皮肤和腹部皮肤)和香腺的组织结构以确定麝香腺是否来源于皮肤。本研究主要得到如下结果:(1)林麝皮肤和香腺组织共同表达的基因高达88.24%,且Metascap预测分析工具证明麝香腺体中表达的基因具有皮肤组织特异性;(2)免疫化学和分子生物实验证实关键调控基因在皮肤和香腺中均有表达;(3)组织形态学分析结果证实在组织空间结构上香腺和皮肤组织中均含有皮脂腺,推测其原因是麝香中主要成分是脂肪酸(71.55%),且皮脂腺和毛囊作为一个结构单元参与林麝信息素的分泌。
Keywords: Forest musk deer; Musk gland; Pheromones; Sebaceous gland; Skin tissues; Transcriptome.
Figures





Similar articles
-
Characteristics of steroidogenesis-related factors in the musk gland of Chinese forest musk deer (Moschus berezovskii).J Steroid Biochem Mol Biol. 2021 Sep;212:105916. doi: 10.1016/j.jsbmb.2021.105916. Epub 2021 May 16. J Steroid Biochem Mol Biol. 2021. PMID: 34010686
-
Single-nucleus RNA and ATAC sequencing uncovers the molecular and cellular characteristics in the musk gland of Chinese forest musk deer (Moschus berezovskii).FASEB J. 2023 Feb;37(2):e22742. doi: 10.1096/fj.202201372R. FASEB J. 2023. PMID: 36583723
-
Integrated multi-omics analysis reveals insights into Chinese forest musk deer (Moschus berezovskii) genome evolution and musk synthesis.Front Cell Dev Biol. 2023 May 9;11:1156138. doi: 10.3389/fcell.2023.1156138. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37228656 Free PMC article.
-
[Research progress on musk secretion mechanism of forest musk deer].Zhongguo Zhong Yao Za Zhi. 2014 Dec;39(23):4522-5. Zhongguo Zhong Yao Za Zhi. 2014. PMID: 25911794 Review. Chinese.
-
Chemical compositions and pharmacological activities of natural musk (Moschus) and artificial musk: A review.J Ethnopharmacol. 2022 Feb 10;284:114799. doi: 10.1016/j.jep.2021.114799. Epub 2021 Nov 6. J Ethnopharmacol. 2022. PMID: 34748869 Review.
Cited by
-
Histological and histochemical characterization of the musk gland in forest musk deer (Moschus berezovskii): a preliminary study.Eur J Histochem. 2025 Jun 17;69(3):4216. doi: 10.4081/ejh.2025.4216. Epub 2025 Jul 10. Eur J Histochem. 2025. PMID: 40637047 Free PMC article.
References
-
- Andrews S, 2014. FastQC a quality control tool for high throughput sequence data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

