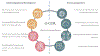
Global Cardio Oncology Registry (G-COR): Registry Design, Primary Objectives, and Future Perspectives of a Multicenter Global Initiative
- PMID: 37702048
- PMCID: PMC10824596
- DOI: 10.1161/CIRCOUTCOMES.123.009905
Global Cardio Oncology Registry (G-COR): Registry Design, Primary Objectives, and Future Perspectives of a Multicenter Global Initiative
Abstract
Background: Global collaboration in cardio-oncology is needed to understand the prevalence of cancer therapy-related cardiovascular toxicity in different risk groups, practice settings, and geographic locations. There are limited data on the socioeconomic and racial/ethnic disparities that may impact access to care and outcomes. To address these gaps, we established the Global Cardio-Oncology Registry, a multinational, multicenter prospective registry.
Methods: We assembled cardiologists and oncologists from academic and community settings to collaborate in the first Global Cardio-Oncology Registry. Subsequently, a survey for site resources, demographics, and intention to participate was conducted. We designed an online data platform to facilitate this global initiative.
Results: A total of 119 sites responded to an online questionnaire on their practices and main goals of the registry: 49 US sites from 23 states and 70 international sites from 5 continents indicated a willingness to participate in the Global Cardio-Oncology Registry. Sites were more commonly led by cardiologists (85/119; 72%) and were more often university/teaching (81/119; 68%) than community based (38/119; 32%). The average number of cardio-oncology patients treated per month was 80 per site. The top 3 Global Cardio-Oncology Registry priorities in cardio-oncology care were breast cancer, hematologic malignancies, and patients treated with immune checkpoint inhibitors. Executive and scientific committees and specific committees were established. A pilot phase for breast cancer using Research Electronic Data Capture Cloud platform recently started patient enrollment.
Conclusions: We present the structure for a global collaboration. Information derived from the Global Cardio-Oncology Registry will help understand the risk factors impacting cancer therapy-related cardiovascular toxicity in different geographic locations and therefore contribute to reduce access gaps in cardio-oncology care. Risk calculators will be prospectively derived and validated.
Keywords: cardiology; cardiotoxicity; cardiovascular disease; hematology; myocarditis.
Conflict of interest statement
Figures



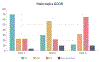
Comment in
-
From Manual to Modern: Accelerating Health Care Transformation With Automatized Electronic Medical Record Registries.Circ Cardiovasc Qual Outcomes. 2023 Oct;16(10):e010379. doi: 10.1161/CIRCOUTCOMES.123.010379. Epub 2023 Sep 13. Circ Cardiovasc Qual Outcomes. 2023. PMID: 37702049 No abstract available.
References
-
- Alexandre J, Cautela J, Ederhy S, Damaj GL, Salem J, Barlesi F, Farnault L, Charbonnier A, Mirabel M, Champiat S, et al. Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the American and European Cardio-Oncology Guidelines. J Am Heart Assoc. 2020;9:e018403. doi: 10.1161/JAHA.120.018403 - DOI - PMC - PubMed
-
- Lancellotti P, Suter TM, López-Fernández T, Galderisi M, Lyon AR, van der Meer P, Cohen Solal A, Zamorano J-L, Jerusalem G, Moonen M, et al. Cardio-Oncology Services: rationale, organization, and implementation: a report from the ESC Cardio-Oncology council. Eur Heart J. 2019;40:1756–1763. doi: 10.1093/eurheartj/ehy453 - DOI - PubMed
-
- Teske AJ, Linschoten M, Kamphuis JAM, Naaktgeboren WR, Leiner T, van der Wall E, Kuball J, van Rhenen A, Doevendans PA, Cramer MJ, et al. Cardio-oncology: an overview on outpatient management and future developments. Netherlands Heart Journal. 2018;26:521–532. doi: 10.1007/s12471-018-1148-7 - DOI - PMC - PubMed

