Stromal matrix directs corneal fibroblasts to re-express keratocan after injury and transplantation
- PMID: 37702214
- PMCID: PMC10508697
- DOI: 10.1242/dmm.050090
Stromal matrix directs corneal fibroblasts to re-express keratocan after injury and transplantation
Abstract
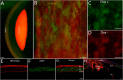
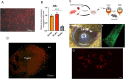
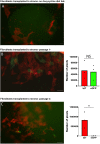
Every tissue has an extracellular matrix (ECM) with certain properties unique to it - the tissue 'niche' - that are necessary for normal function. A distinct specific population of quiescent keratocan-expressing keratocytes populate the corneal stroma during homeostasis to maintain corneal function. However, during wound healing, when there is alteration of the niche conditions, keratocytes undergo apoptosis, and activated corneal fibroblasts and myofibroblasts attempt to restore tissue integrity and function. It is unknown what the fate of activated and temporary fibroblasts and myofibroblasts is after the wound healing process has resolved. In this study, we used several strategies to elucidate the cellular dynamics of corneal wound healing and the fate of corneal fibroblasts. We injured the cornea of a novel mouse model that allows cell-lineage tracing, and we transplanted a cell suspension of in vitro-expanded corneal fibroblasts that could be tracked after being relocated into normal stroma. These transplanted fibroblasts regained expression of keratocan in vivo when relocated to a normal stromal niche. These findings suggest that transformed fibroblasts maintain plasticity and can be induced to a keratocyte phenotype once relocated to an ECM with normal signaling ECM.
Keywords: Keratocan; Keratocyte; Stroma; Transplantation.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests E.M.E. is a consultant with GSK. The remaining authors declare no competing or financial interests.
Figures


References
-
- Beales, M. P., Funderburgh, J. L., Jester, J. V. and Hassell, J. R. (1999). Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: maintenance of the keratocyte phenotype in culture. Invest. Ophthalmol. Vis. Sci. 40, 1658-1663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

