Dose-Dependent Testicular Injury and Recovery after Total-Body Irradiation in Rhesus Monkeys
- PMID: 37702414
- PMCID: PMC10686015
- DOI: 10.1667/RADE-23-00008.1.S1
Dose-Dependent Testicular Injury and Recovery after Total-Body Irradiation in Rhesus Monkeys
Abstract
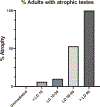
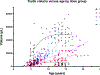
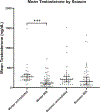
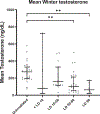
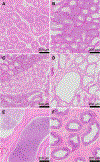
Testicular injury is a well-documented acute effect of radiation exposure, though little is known about recovery years after irradiation, especially at higher doses. We examined the testes from 143 irradiated and control male rhesus monkeys, who were part of the Radiation Late Effects Cohort over a four-year period. Irradiated animals were exposed to doses ranging from 3.5 to 8.5 Gy of total-body irradiation. The testes were assessed using computed tomography (CT) volumetry, serum testosterone, and histology for deceased members of the cohort. Irradiated animals exhibited dose-dependent testicular atrophy as well as decreased serum testosterone during the winter breeding season when compared to age-matched unirradiated controls. No significant difference in summer testosterone levels was observed. Volumetric and histologic evidence of testicular recovery was present approximately three years postirradiation for animals who received ≤8 Gy. The study demonstrates dose-dependent testicular injury after total-body irradiation and provides evidence for volumetric and spermatogonial recovery even at lethal doses of total-body irradiation in rhesus monkeys.
©2023 by Radiation Research Society. All rights of reproduction in any form reserved.
Figures


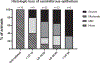
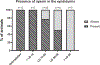
Similar articles
-
Long-term effects of irradiation before adulthood on reproductive function in the male rhesus monkey.Biol Reprod. 2002 Feb;66(2):486-94. doi: 10.1095/biolreprod66.2.486. Biol Reprod. 2002. PMID: 11804966
-
Irradiation affects germ and somatic cells in prepubertal monkey testis xenografts.Mol Hum Reprod. 2017 Mar 1;23(3):141-154. doi: 10.1093/molehr/gax003. Mol Hum Reprod. 2017. PMID: 28130393
-
Irradiation causes acute and long-term spermatogonial depletion in cultured and xenotransplanted testicular tissue from juvenile nonhuman primates.Endocrinology. 2007 Nov;148(11):5541-8. doi: 10.1210/en.2007-0809. Epub 2007 Jul 26. Endocrinology. 2007. PMID: 17656457
-
The testicular transcriptome associated with spermatogonia differentiation initiated by gonadotrophin stimulation in the juvenile rhesus monkey (Macaca mulatta).Hum Reprod. 2017 Oct 1;32(10):2088-2100. doi: 10.1093/humrep/dex270. Hum Reprod. 2017. PMID: 28938749 Free PMC article.
-
Effects of radiation therapy and chemotherapy on testicular function.Prog Clin Biol Res. 1989;302:157-71; discussion 172-7. Prog Clin Biol Res. 1989. PMID: 2666987 Review.
References
-
- Authors on behalf of ICRP, Stewart FA, Akleyev AV, Hauer-Jensen M, Hendry JH, Kleiman NJ, et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs–threshold doses for tissue reactions in a radiation protection context. Ann ICRP. 2012; 41(1–2):1–322. - PubMed
-
- Boehmer D, Badakhshi H, Kuschke W, Bohsung J, Budach V. Testicular dose in prostate cancer radiotherapy: impact on impairment of fertility and hormonal function. Strahlenther Onkol. 2005; 181(3):179–84. - PubMed
-
- Oakes WR, Lushbaugh CC. Course of testicular injury following accidental exposure to nuclear radiations; report of a case. Radiology. 1952; 59(5):737–43. - PubMed
-
- Levison V The effect on fertility, libido and sexual function of post-operative radiotherapy and chemotherapy for cancer of the testicle. Clin Radiol. 1986; 37(2):161–4. - PubMed
-
- Charny CW. Low Dosage Irradiation in Male Infertility - a Negative Report. Fertility and Sterility. 1950; 1(2):150–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

