Evaluation of the QIAstat-Dx Meningitis/Encephalitis Panel, a multiplex PCR platform for the detection of community-acquired meningoencephalitis
- PMID: 37702495
- PMCID: PMC10595057
- DOI: 10.1128/jcm.00426-23
Evaluation of the QIAstat-Dx Meningitis/Encephalitis Panel, a multiplex PCR platform for the detection of community-acquired meningoencephalitis
Abstract
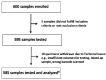
Rapid identification of the causative pathogens of central nervous system infections is essential for providing appropriate management and improving patient outcomes. The performance of QIAstat-Dx Meningitis/Encephalitis (ME) Panel-a multiplex PCR testing platform-in detecting pathogens implicated in meningitis and/or encephalitis was evaluated using BioFire FilmArray ME Panel as a comparator method. This multicenter study analyzed 585 retrospective residual cerebrospinal fluid specimens and 367 contrived specimens. The QIAstat-Dx ME Panel showed positive percent agreement (PPA) values of 100% for Neisseria meningitidis, Streptococcus agalactiae, Escherichia coli K1, Listeria monocytogenes, and Cryptococcus gattii/neoformans on clinical samples compared to the BioFire FilmArray ME Panel. The PPA values observed for Haemophilus influenzae and Streptococcus pneumoniae were 80% and 88.24%, respectively. Negative percent agreement (NPA) values were >99.0% for each of the six bacterial targets and one fungal target tested with clinical samples. One viral target, herpes simplex virus 1, exhibited a PPA value of 100.0%, while the remaining viral targets-human parechovirus, herpes simplex virus 2, human herpes virus 6, and varicella zoster virus-were >90.0%, with the exception of enterovirus, which had a PPA value of 77.8%. The QIAstat-Dx ME Panel detected five true-positive and four true-negative cases compared to BioFire FilmArray ME Panel. The NPA values for all viral pathogens were >99.0%. Overall, the QIAstat-Dx ME Panel showed comparable performance to the BioFire FilmArray ME Panel as a rapid diagnostic tool for community-acquired meningitis and encephalitis.
Keywords: QIAstat-Dx; encephalitis; meningitis; molecular diagnostics; multiplex; real-time PCR.
Conflict of interest statement
QIAGEN provided support in the form of salaries for M.J.F., M.R., S.J., and J.P. T.J. was contracted by QIAGEN for clinical consulting. S.J. declares QIAGEN stock or stock option ownership. Y.C., L.P., J.B., M.H., J.D.D., H.L., and T.S. declare that their institution received financial support and study materials from QIAGEN to perform the study and that QIAGEN provided clinical writing support. T.S. declares to have received paid travel expenses by QIAGEN for a user meeting.
Figures
References
-
- Zunt JR, Kassebaum NJ, Blake N, Glennie L, Wright C, Nichols E, Abd-Allah F, Abdela J, Abdelalim A, Adamu AA, Adib MG, Ahmadi A, Ahmed MB, Aichour AN, Aichour I, Aichour MTE, Akseer N, Al-Raddadi RM, Alahdab F, Alene KA, Aljunid SM, AlMazroa MA, Altirkawi K, Alvis-Guzman N, Animut MD, Anjomshoa M, Ansha MG, Asghar RJ, Avokpaho E, Awasthi A, Badali H, Barac A, Bärnighausen TW, Bassat Q, Bedi N, Belachew AB, Bhattacharyya K, Bhutta ZA, Bijani A, Butt ZA, Carvalho F, Castañeda-Orjuela CA, Chitheer A, Choi J-Y, Christopher DJ, Dang AK, Daryani A, Demoz GT, Djalalinia S, Do HP, Dubey M, Dubljanin E, Duken EE, El Sayed Zaki M, Elyazar IR, Fakhim H, Fernandes E, Fischer F, Fukumoto T, Ganji M, Gebre AK, Gebremeskel A, Gessner BD, Gopalani SV, Guo Y, Gupta R, Hailu GB, Haj-Mirzaian A, Hamidi S, Hay SI, Henok A, Irvani SSN, Jha RP, Jürisson M, Kahsay A, Karami M, Karch A, Kasaeian A, Kassa GM, Kassa TDD, Kefale AT, Khader YS, Khalil IA, Khan EA, Khang Y-H, Khubchandani J, Kimokoti RW, Kisa A, Lami FH, Levi M, Li S, Loy CT, Majdan M, Majeed A, Mantovani LG, Martins-Melo FR, Mcalinden C, Mehta V, Melese A, Memish ZA, Mengistu DT, Mengistu G, Mestrovic T, Mezgebe HB, Miazgowski B, Milosevic B, Mokdad AH, Monasta L, Moradi G, Moraga P, Mousavi SM, Mueller UO, Murthy S, Mustafa G, Naghavi M, Naheed A, Naik G, Newton CRJ, Nirayo YL, Nixon MR, Ofori-Asenso R, Ogbo FA, Olagunju AT, Olagunju TO, Olusanya BO, Ortiz JR, Owolabi MO, Patel S, Pinilla-Monsalve GD, Postma MJ, Qorbani M, Rafiei A, Rahimi-Movaghar V, Reiner RC, Renzaho AMN, Rezai MS, Roba KT, Ronfani L, Roshandel G, Rostami A, Safari H, Safari S, Safiri S, Sagar R, Samy AM, Santric Milicevic MM, Sartorius B, Sarvi S, Sawhney M, Saxena S, Shafieesabet A, Shaikh MA, Sharif M, Shigematsu M, Si S, Skiadaresi E, Smith M, Somayaji R, Sufiyan MB, Tawye NY, Temsah M-H, Tortajada-Girbés M, Tran BX, Tran KB, Ukwaja KN, Ullah I, Vujcic IS, Wagnew FS, Waheed Y, Weldegwergs KG, Winkler AS, Wiysonge CS, Wiyeh AB, Wyper GMA, Yimer EM, Yonemoto N, Zaidi Z, Zenebe ZM, Feigin VL, Vos T, Murray CJL. 2018. Global, regional, and national burden of meningitis, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 17:1061–1082. doi:10.1016/S1474-4422(18)30387-9 - DOI - PMC - PubMed
-
- McGill F, Heyderman RS, Michael BD, Defres S, Beeching NJ, Borrow R, Glennie L, Gaillemin O, Wyncoll D, Kaczmarski E, Nadel S, Thwaites G, Cohen J, Davies NWS, Miller A, Rhodes A, Read RC, Solomon T. 2016. The UK joint specialist societies guideline on the diagnosis and management of acute meningitis and meningococcal sepsis in immunocompetent adults. J Infect 72:405–438. doi:10.1016/j.jinf.2016.01.007 - DOI - PubMed
-
- Tunkel AR, Glaser CA, Bloch KC, Sejvar JJ, Marra CM, Roos KL, Hartman BJ, Kaplan SL, Scheld WM, Whitley RJ, Infectious Diseases Society of America . 2008. The management of encephalitis: clinical practice guidelines by the infectious diseases society of America. Clin Infect Dis 47:303–327. doi:10.1086/589747 - DOI - PubMed
-
- van de Beek D, Cabellos C, Dzupova O, Esposito S, Klein M, Kloek AT, Leib SL, Mourvillier B, Ostergaard C, Pagliano P, Pfister HW, Read RC, Sipahi OR, Brouwer MC, ESCMID Study Group for Infections of the Brain (ESGIB) . 2016. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect 22:S37–S62. doi:10.1016/j.cmi.2016.01.007 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

