A phase II study of bevacizumab and temsirolimus in advanced extra-pancreatic neuroendocrine tumors
- PMID: 37702588
- PMCID: PMC10585708
- DOI: 10.1530/ERC-22-0301
A phase II study of bevacizumab and temsirolimus in advanced extra-pancreatic neuroendocrine tumors
Abstract
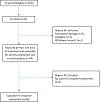
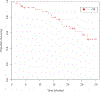
We assessed the efficacy and safety of combining bevacizumab with temsirolimus in patients with advanced extra-pancreatic neuroendocrine tumors. This NCI-sponsored multicenter, open-label, phase II study (NCT01010126) enrolled patients with advanced, recurrent, or metastatic extra-pancreatic neuroendocrine tumors. All patients were treated with temsirolimus and bevacizumab until disease progression or unacceptable toxicity. Temsirolimus 25 mg was administered i.v. on days 1, 8, 15, and 22 and bevacizumab 10 mg/kg i.v. on days 1 and 15 of a 4-week cycle. Discontinuation of temsirolimus or bevacizumab did not require discontinuation of the other agent. The primary endpoints were objective response rate and 6-month progression-free survival rate. Fifty-nine patients were enrolled in this study, and 54 were evaluated for efficacy and adverse events. While median progression-free survival was 7.1 months, the median duration of treatment with temsirolimus was 3.9 months and that with bevacizumab was 3.5 months. The objective response rate of combination therapy was 2%, and 6-month progression-free survival was 48%. The most frequently reported grade 3-4 adverse events included fatigue (13%), hypertension (13%), and bleeding (13%). Close to 54% of the patients discontinued treatment due to adverse events, refusal of further treatment, or treatment delays. Three deaths occurred in the study, of which two were due to treatment-related bowel perforations. Given the minimal efficacy and increased toxicity seen with the combination of bevacizumab and temsirolimus, we do not recommend the use of this regimen in patients with advanced extra-pancreatic neuroendocrine tumors.
Keywords: bevacizumab; carcinoid; mTOR inhibitor; neuroendocrine; temsirolimus.
Conflict of interest statement
Declaration of Interest
Jonathan Strosberg: Honoraria from Speaker’s Bureau from Genentech; Jennifer J. Knox: Commercial research support from Pfizer for an investigator-initiated study; Manisha Shah: Commercial research grant from Novartis and consult/advisory board for Novartis; Vineeth Sukrithan: Research funding (to institution) from Eli Lilly & Company, consulting fees from GE Health and Lantheus. The remaining authors have no conflicts to disclose.
Figures
References
-
- BANCK MS, KANWAR R, KULKARNI AA, BOORA GK, METGE F, KIPP BR, ZHANG L, THORLAND EC, MINN KT, TENTU R, ECKLOFF BW, WIEBEN ED, WU Y, CUNNINGHAM JM, NAGORNEY DM, GILBERT JA, AMES MM & BEUTLER AS 2013. The genomic landscape of small intestine neuroendocrine tumors. J Clin Invest, 123, 2502–8. - PMC - PubMed
-
- CAPELLA C SE, SOBIN LH et al. ENDOCRINE TUMOURS OF THE SMALL INTESTINE. IN: HAMILTON SR, AALTONEN LA, EDS. PATHOLOGY AND GENETICS OF TUMOURS OF THE DIGESTIVE SYSTEM. LYON, FRANCE: IARC PRESS; 2000:77–82.
-
- CAPLIN ME, PAVEL M, ĆWIKŁA JB, PHAN AT, RADERER M, SEDLÁČKOVÁ E, CADIOT G, WOLIN EM, CAPDEVILA J, WALL L, RINDI G, LANGLEY A, MARTINEZ S, BLUMBERG J & RUSZNIEWSKI P 2014. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. New England Journal of Medicine, 371, 224–233. - PubMed
-
- DURAN I, KORTMANSKY J, SINGH D, HIRTE H, KOCHA W, GOSS G, LE L, OZA A, NICKLEE T, HO J, BIRLE D, POND GR, ARBOINE D, DANCEY J, AVIEL-RONEN S, TSAO MS, HEDLEY D & SIU LL 2006. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer, 95, 1148–54. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous