Autoimmune lymphoproliferative immunodeficiencies (ALPID) in childhood: breakdown of immune homeostasis and immune dysregulation
- PMID: 37702894
- PMCID: PMC10499775
- DOI: 10.1186/s40348-023-00167-1
Autoimmune lymphoproliferative immunodeficiencies (ALPID) in childhood: breakdown of immune homeostasis and immune dysregulation
Abstract
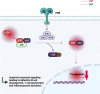
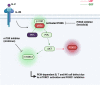
Many inborn errors of immunity (IEI) manifest with hallmarks of both immunodeficiency and immune dysregulation due to uncontrolled immune responses and impaired immune homeostasis. A subgroup of these disorders frequently presents with autoimmunity and lymphoproliferation (ALPID phenotype). After the initial description of the genetic basis of autoimmune lymphoproliferative syndrome (ALPS) more than 20 years ago, progress in genetics has helped to identify many more genetic conditions underlying this ALPID phenotype. Among these, the majority is caused by a group of autosomal-dominant conditions including CTLA-4 haploinsufficiency, STAT3 gain-of-function disease, activated PI3 kinase syndrome, and NF-κB1 haploinsufficiency. Even within a defined genetic condition, ALPID patients may present with staggering clinical heterogeneity, which makes diagnosis and management a challenge. In this review, we discuss the pathophysiology, clinical presentation, approaches to diagnosis, and conventional as well as targeted therapy of the most common ALPID conditions.
Keywords: Autoimmune lymphoproliferative immunodeficiencies; Immune dysregulation; Inborn errors of immunity; Pathogenesis; Targeted therapy.
© 2023. Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

