Assessment of symptoms in COMET-ICE, a phase 2/3 study of sotrovimab for early treatment of non-hospitalized patients with COVID-19
- PMID: 37702920
- PMCID: PMC10499766
- DOI: 10.1186/s41687-023-00621-8
Assessment of symptoms in COMET-ICE, a phase 2/3 study of sotrovimab for early treatment of non-hospitalized patients with COVID-19
Abstract
Background: The COMET-ICE trial demonstrated that sotrovimab clinically and statistically significantly reduces the risk of all-cause > 24-h hospitalization or death due to any cause among patients with COVID-19 at high risk of disease progression. Patient-reported outcomes are important to capture symptom burden of COVID-19 and assess treatment effectiveness. This study investigated symptoms and their impact over the acute phase of COVID-19 infection among patients on sotrovimab versus placebo.
Methods: Randomized (1:1), double-blind, multicenter, placebo-controlled, phase 2/3 study in 57 centers across five countries. Participants were non-hospitalized patients with symptomatic, mild-to-moderate COVID-19 and ≥ 1 baseline risk factor for disease progression (aged ≥ 55 years or ≥ 1 of the following: diabetes requiring medication, obesity, chronic kidney disease, congestive heart failure, chronic obstructive pulmonary disease, or moderate-to-severe asthma). An intravenous infusion of sotrovimab 500 mg or placebo was administered on Day 1. The FLU-PRO Plus questionnaire was administered once-daily with 24-h recall from Day 1-21, and at Day 29. Intensity and duration of COVID-19 symptoms were determined from area under the curve (AUC) and mean change in total and individual domain scores through Days 7, 14, and 21. Time to symptom alleviation was assessed.
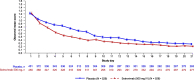
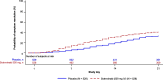
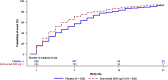
Results: In total, 1057 patients were randomized to sotrovimab (n = 528) or placebo (n = 529). At Day 7, mean decrease in FLU-PRO Plus total score (measured by AUC) was statistically significantly greater for patients on sotrovimab (-3.05 [95% confidence interval (CI) -3.27 to -2.83]) than placebo (-1.98 [95% CI -2.20 to -1.76]; difference -1.07 [95% CI -1.38 to -0.76]; p < 0.001). Significant differences were also observed at Days 14 and 21. A more rapid decline in symptom severity was observed with sotrovimab versus placebo through Week 1 and the first 21 days post-treatment. By Day 21, 41% of patients on sotrovimab and 34% on placebo reported symptom resolution. In a post-hoc analysis, median time to symptom alleviation was 4 and 6 days, respectively.
Conclusions: Sotrovimab provides significant and rapid improvements in patient-reported COVID-19 symptoms, as measured by the FLU-PRO Plus. These results further show the benefits of sotrovimab in alleviating symptoms among high-risk patients with COVID-19. Trial registration ClinicalTrials.Gov: NCT04545060 ( https://clinicaltrials.gov/ct2/show/NCT04545060 ). Date of registration: September 10, 2020 (retrospectively registered).
Keywords: COVID-19; FLU-PRO Plus; Monoclonal antibody; PRO; Patient-reported outcomes; Sotrovimab; Symptoms.
© 2023. International Society for Quality of Life Research (ISOQOL).
Conflict of interest statement
PG, TJHK, HJB, DB, and AL are employees of, and/or hold stocks/shares in, GSK. SS, MA, and EA are employees of, and/or hold stocks/shares in, Vir Biotechnology, Inc. CMR was an employee of Vir Biotechnology, Inc. at the time of study. JHP III has consulted for Arrevus, Eicos, Eli Lilly, Evofem, Eyecheck, Fuji, Gilead, Johnson & Johnson, Microbion, OPKO, Otsuka, Resolve, Romark, Shinogi, SpineBioPharma, and UTIlity, outside the submitted work, and for GSK and Vir Biotechnology, Inc. for the submitted work. AG and AES have acted as trial investigators for Vir Biotechnology, Inc. and received non-financial support from Vir Biotechnology, Inc. during the conduct of the study. EHS has acted as a trial investigator for Vir Biotechnology, Inc. and received non-financial support from Vir Biotechnology, Inc. during the conduct of the study; has received research support from AbbVie, Eisai, Eli Lilly, GSK, Ironshore, Otsuka, and Vir Biotechnology, Inc.; and served on speaker bureaus for AbbVie, Janssen, and Teva.
Figures
References
-
- Cathcart AL, Havenar-Daughton C, Lempp FA et al (2022) The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv 2021.2003.2009.434607. 10.1101/2021.03.09.434607
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

