Trazodone rescues dysregulated synaptic and mitochondrial nascent proteomes in prion neurodegeneration
- PMID: 37703312
- PMCID: PMC10834243
- DOI: 10.1093/brain/awad313
Trazodone rescues dysregulated synaptic and mitochondrial nascent proteomes in prion neurodegeneration
Abstract
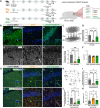
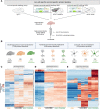
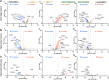
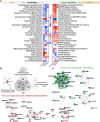
The unfolded protein response (UPR) is rapidly gaining momentum as a therapeutic target for protein misfolding neurodegenerative diseases, in which its overactivation results in sustained translational repression leading to synapse loss and neurodegeneration. In mouse models of these disorders, from Alzheimer's to prion disease, modulation of the pathway-including by the licensed drug, trazodone-restores global protein synthesis rates with profound neuroprotective effects. However, the precise nature of the translational impairment, in particular the specific proteins affected in disease, and their response to therapeutic UPR modulation are poorly understood. We used non-canonical amino acid tagging (NCAT) to measure de novo protein synthesis in the brains of prion-diseased mice with and without trazodone treatment, in both whole hippocampus and cell-specifically. During disease the predominant nascent proteome changes occur in synaptic, cytoskeletal and mitochondrial proteins in both hippocampal neurons and astrocytes. Remarkably, trazodone treatment for just 2 weeks largely restored the whole disease nascent proteome in the hippocampus to that of healthy, uninfected mice, predominantly with recovery of proteins involved in synaptic and mitochondrial function. In parallel, trazodone treatment restored the disease-associated decline in synapses and mitochondria and their function to wild-type levels. In conclusion, this study increases our understanding of how translational repression contributes to neurodegeneration through synaptic and mitochondrial toxicity via depletion of key proteins essential for their function. Further, it provides new insights into the neuroprotective mechanisms of trazodone through reversal of this toxicity, relevant for the treatment of neurodegenerative diseases via translational modulation.
Keywords: UPR/ISR; mitochondria; nascent proteome; neurodegeneration; synapses; translational repression; trazodone.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures

References
-
- Hoozemans JJM, Veerhuis R, Van Haastert ES, et al. The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol. 2005;110(2):165–172. - PubMed
-
- Hoozemans JJM, Van Haastert ES, Eikelenboom JJM, de Vos RAI, Rozemuller JM, Scheper W. Activation of the unfolded protein response in Parkinson’s disease. Biochem Biophys Res Commun. 2007;354:707–711. - PubMed
-
- Nijholt DAT, Van Haastert ES, Rozemuller AJM, Scheper W, Hoozemans JJM. The unfolded protein response is associated with early tau pathology in the hippocampus of tauopathies. J Pathol. 2012;226:693–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

