Chimeric hemagglutinin split vaccines elicit broadly cross-reactive antibodies and protection against group 2 influenza viruses in mice
- PMID: 37703367
- PMCID: PMC10499326
- DOI: 10.1126/sciadv.adi4753
Chimeric hemagglutinin split vaccines elicit broadly cross-reactive antibodies and protection against group 2 influenza viruses in mice
Abstract
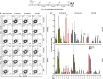
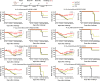
Seasonal influenza virus vaccines are effective when they are well matched to circulating strains. Because of antigenic drift/change in the immunodominant hemagglutinin (HA) head domain, annual vaccine reformulations are necessary to maintain a match with circulating strains. In addition, seasonal vaccines provide little to no protection against newly emerging pandemic strains. Sequential vaccination with chimeric HA (cHA) constructs has been proven to direct the immune response toward the immunosubdominant but more conserved HA stalk domain. In this study, we show that immunization with group 2 cHA split vaccines in combination with the CpG 1018 adjuvant elicits broadly cross-reactive antibodies against all group 2 HAs, as well as systemic and local antigen-specific T cell responses. Antibodies elicited after sequential vaccination are directed to conserved regions of the HA such as the stalk and the trimer interface and also to the N2 neuraminidase (NA). Immunized mice were fully protected from challenge with a broad panel of influenza A viruses.
Figures



References
-
- E. A. Belongia, H. Q. McLean, Influenza Vaccine Effectiveness: Defining the H3N2 Problem. Clin. Infect. Dis. 69, 1817–1823 (2019). - PubMed
-
- G. N. Okoli, F. Racovitan, T. Abdulwahid, C. H. Righolt, S. M. Mahmud, Variable seasonal influenza vaccine effectiveness across geographical regions, age groups and levels of vaccine antigenic similarity with circulating virus strains: A systematic review and meta-analysis of the evidence from test-negative design studies after the 2009/10 influenza pandemic. Vaccine 39, 1225–1240 (2021). - PubMed
-
- Y. Suzuki, Positive selection for gains of N-linked glycosylation sites in hemagglutinin during evolution of H3N2 human influenza A virus. Genes Genet. Syst. 86, 287–294 (2011). - PubMed
-
- S. Rajaram, R. Wojcik, C. Moore, R. Ortiz de Lejarazu, S. de Lusignan, E. Montomoli, A. Rossi, A. Pérez-Rubio, A. Trilla, V. Baldo, R. Jandhyala, G. Kassianos, The impact of candidate influenza virus and egg-based manufacture on vaccine effectiveness: Literature review and expert consensus. Vaccine 38, 6047–6056 (2020). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

