Tolerability and Efficacy of the Anticluster of Differentiation 47 Antibody Magrolimab Combined With Azacitidine in Patients With Previously Untreated AML: Phase Ib Results
- PMID: 37703506
- PMCID: PMC10617926
- DOI: 10.1200/JCO.22.02604
Tolerability and Efficacy of the Anticluster of Differentiation 47 Antibody Magrolimab Combined With Azacitidine in Patients With Previously Untreated AML: Phase Ib Results
Abstract
Purpose: Magrolimab is a first-in-class humanized monoclonal antibody against cluster of differentiation 47, an antiphagocytic signal used by cancer cells to evade phagocytosis. Azacitidine upregulates prophagocytic signals on AML cells, further increasing phagocytosis when combined with magrolimab. We report final phase Ib data for magrolimab with azacitidine in patients with untreated AML ineligible for intensive chemotherapy (ClinicalTrials.gov identifier: NCT03248479).
Patients and methods: Patients with previously untreated AML, including TP53-mutant AML, received magrolimab intravenously as an initial dose (1 mg/kg, days 1 and 4), followed by 15 mg/kg once on day 8 and 30 mg/kg once weekly or every 2 weeks as maintenance. Azacitidine 75 mg/m2 was administered intravenously/subcutaneously once daily on days 1-7 of each 28-day cycle. Primary end points were safety/tolerability and proportion with complete remission (CR).
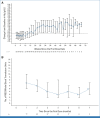
Results: Eighty-seven patients were enrolled and treated; 72 (82.8%) had TP53 mutations with a median variant allele frequency of 61% (range, 9.8-98.7). Fifty-seven (79.2%) of TP53-mutant patients had European LeukemiaNet 2017 adverse-risk cytogenetics. Patients received a median of 4 (range, 1-39) cycles of treatment. The most common treatment-emergent adverse events included constipation (49.4%), nausea (49.4%), and diarrhea (48.3%). Thirty (34.5%) experienced anemia, and the median hemoglobin change from baseline to first postdose assessment was -0.9 g/dL (range, -3.6 to 2.5 g/dL). Twenty-eight (32.2%) patients achieved CR, including 23 (31.9%) patients with TP53 mutations. The median overall survival in TP53-mutant and wild-type patients were 9.8 months and 18.9 months, respectively.
Conclusion: Magrolimab with azacitidine was relatively well tolerated with promising efficacy in patients with AML ineligible for intensive induction chemotherapy, including those with TP53 mutations, warranting further evaluation of magrolimab with azacitidine in AML. The phase III randomized ENHANCE-2 (ClinicalTrials.gov identifier: NCT04778397) and ENHANCE-3 (ClinicalTrials.gov identifier: NCT05079230) studies are recruiting frontline patients with AML.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures


References
-
- Kantarjian H, O'Brien S, Cortes J, et al. : Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer 106:1090-1098, 2006 - PubMed
-
- National Cancer Institute : Surveillance, Epidemiology, and End Results Program: Acute myeloid leukemia (AML) SEER 5-year relative survival rates, 2013-2019. https://seer.cancer.gov/statistics-network/explorer/application.html?si...
-
- Tsai CH, Hou HA, Tang JL, et al. : Genetic alterations and their clinical implications in older patients with acute myeloid leukemia. Leukemia 30:1485-1492, 2016 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

