New perspectives on the causes and consequences of male meiotic drive
- PMID: 37704518
- PMCID: PMC10842977
- DOI: 10.1016/j.gde.2023.102111
New perspectives on the causes and consequences of male meiotic drive
Abstract
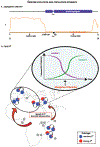
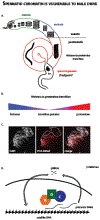
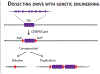
Gametogenesis is vulnerable to selfish genetic elements that bias their transmission to the next generation by cheating meiosis. These so-called meiotic drivers are widespread in plants, animals, and fungi and can impact genome evolution. Here, we summarize recent progress on the causes and consequences of meiotic drive in males, where selfish elements attack vulnerabilities in spermatogenesis. Advances in genomics provide new insights into the organization and dynamics of driving chromosomes in natural populations. Common themes, including small RNAs, gene duplications, and heterochromatin, emerged from these studies. Interdisciplinary approaches combining evolutionary genomics with molecular and cell biology are beginning to unravel the mysteries of drive and suppression mechanisms. These approaches also provide insights into fundamental processes in spermatogenesis and chromatin regulation.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest We have no conflicts of interest to declare.
Figures

References
-
- Lindholm AK, Dyer KA, Firman RC, Fishman L, Forstmeier W, Holman L, Johannesson H, Knief U, Kokko H, Larracuente AM, et al. : The ecology and evolutionary dynamics of meiotic drive. Trends Ecol Evol 2016, 31:315–326. - PubMed
-
- Navarro-Dominguez B, Chang C-H, Brand CL, Muirhead CA, Presgraves DC, Larracuente AM: Epistatic selection on a selfish Segregation Distorter supergene – drive, recombination, and genetic load. eLife 2022, 11:e78981. - PMC - PubMed
-
The authors combine population genomic and genetic approaches to study the evolution of the Segregation Distorter (SD) system of Drosophila melanogaster. They show evidence that SD in African populations corresponds to a supergene that caused a chromosome-scale selective sweep consistent with strong epistatic selection. They also show evidence that gene conversion and rare recombination might prevent the supergene from degenerating like a completely non-recombining chromosome.
-
- Fuller ZL, Koury SA, Leonard CJ, Young RE, Ikegami K, Westlake J, Richards S, Schaeffer SW, Phadnis N: Extensive recombination suppression and epistatic selection causes chromosome-wide differentiation of a selfish sex chromosome in Drosophila pseudoobscura. Genetics 2020, 216:205–226. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

