Human OPRM1 and murine Oprm1 promoter driven viral constructs for genetic access to μ-opioidergic cell types
- PMID: 37704594
- PMCID: PMC10499891
- DOI: 10.1038/s41467-023-41407-2
Human OPRM1 and murine Oprm1 promoter driven viral constructs for genetic access to μ-opioidergic cell types
Abstract
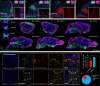
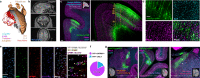
With concurrent global epidemics of chronic pain and opioid use disorders, there is a critical need to identify, target and manipulate specific cell populations expressing the mu-opioid receptor (MOR). However, available tools and transgenic models for gaining long-term genetic access to MOR+ neural cell types and circuits involved in modulating pain, analgesia and addiction across species are limited. To address this, we developed a catalog of MOR promoter (MORp) based constructs packaged into adeno-associated viral vectors that drive transgene expression in MOR+ cells. MORp constructs designed from promoter regions upstream of the mouse Oprm1 gene (mMORp) were validated for transduction efficiency and selectivity in endogenous MOR+ neurons in the brain, spinal cord, and periphery of mice, with additional studies revealing robust expression in rats, shrews, and human induced pluripotent stem cell (iPSC)-derived nociceptors. The use of mMORp for in vivo fiber photometry, behavioral chemogenetics, and intersectional genetic strategies is also demonstrated. Lastly, a human designed MORp (hMORp) efficiently transduced macaque cortical OPRM1+ cells. Together, our MORp toolkit provides researchers cell type specific genetic access to target and functionally manipulate mu-opioidergic neurons across a range of vertebrate species and translational models for pain, addiction, and neuropsychiatric disorders.
© 2023. Springer Nature Limited.
Conflict of interest statement
G.C, K.D., C.R. and G.J.S. are listed as inventors on a provisional patent application filed on November 11th, 2022 through both the University of Pennsylvania and Stanford University regarding the custom sequences used to develop, and the applications of, both the
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
- F31 DA057795/DA/NIDA NIH HHS/United States
- F32 DA053099/DA/NIDA NIH HHS/United States
- P50 HD105351/HD/NICHD NIH HHS/United States
- R37 MH109728/MH/NIMH NIH HHS/United States
- F31 NS125927/NS/NINDS NIH HHS/United States
- F32 DA055458/DA/NIDA NIH HHS/United States
- R01 DK112812/DK/NIDDK NIH HHS/United States
- R00 DA043609/DA/NIDA NIH HHS/United States
- T32 DA028874/DA/NIDA NIH HHS/United States
- DP2 GM140923/GM/NIGMS NIH HHS/United States
- R35 NS105076/NS/NINDS NIH HHS/United States
- R01 DK130239/DK/NIDDK NIH HHS/United States
- DP1 DA054343/DA/NIDA NIH HHS/United States
- R01 NS119476/NS/NINDS NIH HHS/United States
- R01 DA056599/DA/NIDA NIH HHS/United States
- R01 DA054374/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

