Genetically encoded discovery of perfluoroaryl macrocycles that bind to albumin and exhibit extended circulation in vivo
- PMID: 37704629
- PMCID: PMC10499988
- DOI: 10.1038/s41467-023-41427-y
Genetically encoded discovery of perfluoroaryl macrocycles that bind to albumin and exhibit extended circulation in vivo
Erratum in
-
Author Correction: Genetically encoded discovery of perfluoroaryl macrocycles that bind to albumin and exhibit extended circulation in vivo.Nat Commun. 2023 Nov 21;14(1):7587. doi: 10.1038/s41467-023-43517-3. Nat Commun. 2023. PMID: 37990013 Free PMC article. No abstract available.
Abstract
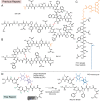
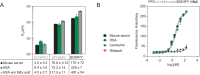
Peptide-based therapeutics have gained attention as promising therapeutic modalities, however, their prevalent drawback is poor circulation half-life in vivo. In this paper, we report the selection of albumin-binding macrocyclic peptides from genetically encoded libraries of peptides modified by perfluoroaryl-cysteine SNAr chemistry, with decafluoro-diphenylsulfone (DFS). Testing of the binding of the selected peptides to albumin identified SICRFFC as the lead sequence. We replaced DFS with isosteric pentafluorophenyl sulfide (PFS) and the PFS-SICRFFCGG exhibited KD = 4-6 µM towards human serum albumin. When injected in mice, the concentration of the PFS-SICRFFCGG in plasma was indistinguishable from the reference peptide, SA-21. More importantly, a conjugate of PFS-SICRFFCGG and peptide apelin-17 analogue (N3-PEG6-NMe17A2) showed retention in circulation similar to SA-21; in contrast, apelin-17 analogue was cleared from the circulation after 2 min. The PFS-SICRFFC is the smallest known peptide macrocycle with a significant affinity for human albumin and substantial in vivo circulation half-life. It is a productive starting point for future development of compact macrocycles with extended half-life in vivo.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare the following competing financial interests. Ratmir Derda is the founder and CEO of 48Hour Discovery Inc. The remaining authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

