Laser-induced nitrogen fixation
- PMID: 37704640
- PMCID: PMC10499830
- DOI: 10.1038/s41467-023-41441-0
Laser-induced nitrogen fixation
Abstract
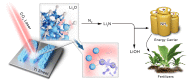
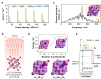
For decarbonization of ammonia production in industry, alternative methods by exploiting renewable energy sources have recently been explored. Nonetheless, they still lack yield and efficiency to be industrially relevant. Here, we demonstrate an advanced approach of nitrogen fixation to synthesize ammonia at ambient conditions via laser-induced multiphoton dissociation of lithium oxide. Lithium oxide is dissociated under non-equilibrium multiphoton absorption and high temperatures under focused infrared light, and the generated zero-valent metal spontaneously fixes nitrogen and forms a lithium nitride, which upon subsequent hydrolysis generates ammonia. The highest ammonia yield rate of 30.9 micromoles per second per square centimeter is achieved at 25 °C and 1.0 bar nitrogen. This is two orders of magnitude higher than state-of-the-art ammonia synthesis at ambient conditions. The focused infrared light here is produced by a commercial simple CO2 laser, serving as a demonstration of potentially solar pumped lasers for nitrogen fixation and other high excitation chemistry. We anticipate such laser-involved technology will bring unprecedented opportunities to realize not only local ammonia production but also other new chemistries .
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Lehnert N, Dong HT, Harland JB, Hunt AP, White CJ. Reversing nitrogen fixation. Nat. Rev. Chem. 2018;2:278–289. doi: 10.1038/s41570-018-0041-7. - DOI
-
- Kyriakou V, Garagounis I, Vourros A, Vasileiou E, Stoukides M. An electrochemical Haber-Bosch. Process. Joule. 2020;4:142–158. doi: 10.1016/j.joule.2019.10.006. - DOI
LinkOut - more resources
Full Text Sources

