A vertebral skeletal stem cell lineage driving metastasis
- PMID: 37704733
- PMCID: PMC10829697
- DOI: 10.1038/s41586-023-06519-1
A vertebral skeletal stem cell lineage driving metastasis
Abstract
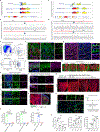
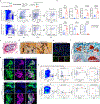
Vertebral bone is subject to a distinct set of disease processes from long bones, including a much higher rate of solid tumour metastases1-4. The basis for this distinct biology of vertebral bone has so far remained unknown. Here we identify a vertebral skeletal stem cell (vSSC) that co-expresses ZIC1 and PAX1 together with additional cell surface markers. vSSCs display formal evidence of stemness, including self-renewal, label retention and sitting at the apex of their differentiation hierarchy. vSSCs are physiologic mediators of vertebral bone formation, as genetic blockade of the ability of vSSCs to generate osteoblasts results in defects in the vertebral neural arch and body. Human counterparts of vSSCs can be identified in vertebral endplate specimens and display a conserved differentiation hierarchy and stemness features. Multiple lines of evidence indicate that vSSCs contribute to the high rates of vertebral metastatic tropism observed in breast cancer, owing in part to increased secretion of the novel metastatic trophic factor MFGE8. Together, our results indicate that vSSCs are distinct from other skeletal stem cells and mediate the unique physiology and pathology of vertebrae, including contributing to the high rate of vertebral metastasis.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Figures













Update of
-
Discovery of a Vertebral Skeletal Stem Cell Driving Spinal Metastases.Res Sq [Preprint]. 2023 Jan 25:rs.3.rs-2106142. doi: 10.21203/rs.3.rs-2106142/v1. Res Sq. 2023. Update in: Nature. 2023 Sep;621(7979):602-609. doi: 10.1038/s41586-023-06519-1. PMID: 36747772 Free PMC article. Updated. Preprint.
Comment in
-
Stem cells provide clues to why vertebrae attract tumour cells.Nature. 2023 Sep;621(7979):481-482. doi: 10.1038/d41586-023-02768-2. Nature. 2023. PMID: 37704844 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

