Revolutionizing control strategies against Mycobacterium tuberculosis infection through selected targeting of lipid metabolism
- PMID: 37704889
- PMCID: PMC11072447
- DOI: 10.1007/s00018-023-04914-5
Revolutionizing control strategies against Mycobacterium tuberculosis infection through selected targeting of lipid metabolism
Abstract
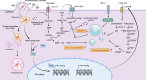
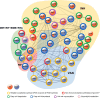
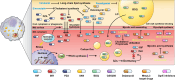
Lipid species play a critical role in the growth and virulence expression of Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB). During Mtb infection, foamy macrophages accumulate lipids in granulomas, providing metabolic adaptation and survival strategies for Mtb against multiple stresses. Host-derived lipid species, including triacylglycerol and cholesterol, can also contribute to the development of drug-tolerant Mtb, leading to reduced efficacy of antibiotics targeting the bacterial cell wall or transcription. Transcriptional and metabolic analyses indicate that lipid metabolism-associated factors of Mtb are highly regulated by antibiotics and ultimately affect treatment outcomes. Despite the well-known association between major antibiotics and lipid metabolites in TB treatment, a comprehensive understanding of how altered lipid metabolites in both host and Mtb influence treatment outcomes in a drug-specific manner is necessary to overcome drug tolerance. The current review explores the controversies and correlations between lipids and drug efficacy in various Mtb infection models and proposes novel approaches to enhance the efficacy of anti-TB drugs. Moreover, the review provides insights into the efficacious control of Mtb infection by elucidating the impact of lipids on drug efficacy. This review aims to improve the effectiveness of current anti-TB drugs and facilitate the development of innovative therapeutic strategies against Mtb infection by making reverse use of Mtb-favoring lipid species.
Keywords: Anti-TB drugs; Drug efficacy; Drug tolerance; Lipid droplet; Lipid metabolism; Mycobacterium tuberculosis.
© 2023. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Editorial: Current status and perspective on drug targets in tubercle bacilli and drug design of antituberculous agents based on structure-activity relationship.Curr Pharm Des. 2014;20(27):4305-6. doi: 10.2174/1381612819666131118203915. Curr Pharm Des. 2014. PMID: 24245755
-
A Novel Tool to Identify Bactericidal Compounds against Vulnerable Targets in Drug-Tolerant M. tuberculosis found in Caseum.mBio. 2023 Apr 25;14(2):e0059823. doi: 10.1128/mbio.00598-23. Epub 2023 Apr 5. mBio. 2023. PMID: 37017524 Free PMC article.
-
Host-directed therapy targeting the Mycobacterium tuberculosis granuloma: a review.Semin Immunopathol. 2016 Mar;38(2):167-83. doi: 10.1007/s00281-015-0537-x. Epub 2015 Oct 28. Semin Immunopathol. 2016. PMID: 26510950 Free PMC article. Review.
-
Harnessing Biological Insight to Accelerate Tuberculosis Drug Discovery.Acc Chem Res. 2019 Aug 20;52(8):2340-2348. doi: 10.1021/acs.accounts.9b00275. Epub 2019 Jul 30. Acc Chem Res. 2019. PMID: 31361123 Free PMC article. Review.
-
The impact of Mycobacterium tuberculosis on the macrophage cholesterol metabolism pathway.Front Immunol. 2024 May 30;15:1402024. doi: 10.3389/fimmu.2024.1402024. eCollection 2024. Front Immunol. 2024. PMID: 38873598 Free PMC article. Review.
Cited by
-
Contribution of infectious diseases to the selection of ADH1B and ALDH2 gene variants in Asian populations.Alcohol Clin Exp Res (Hoboken). 2024 May;48(5):855-866. doi: 10.1111/acer.15288. Epub 2024 Mar 10. Alcohol Clin Exp Res (Hoboken). 2024. PMID: 38462538 Free PMC article.
-
Trimming the fat: a brief review of lipids at the host-pathogen interface.Infect Immun. 2025 Jul 8;93(7):e0050624. doi: 10.1128/iai.00506-24. Epub 2025 Jun 13. Infect Immun. 2025. PMID: 40512027 Free PMC article. Review.
-
Synthesis of cationic N-acylated thiazolidine for selective activity against Gram-positive bacteria and evaluation of N-acylation's role in membrane-disrupting activity.RSC Med Chem. 2024 Oct 21. doi: 10.1039/d4md00626g. Online ahead of print. RSC Med Chem. 2024. PMID: 39507614 Free PMC article.
-
Mendelian randomization study of lipid species reveals causal relationship with syphilis.AMB Express. 2025 Apr 9;15(1):63. doi: 10.1186/s13568-025-01873-x. AMB Express. 2025. PMID: 40205291 Free PMC article.
-
Development and Validation of Early Alert Model for Diabetes Mellitus-Tuberculosis Comorbidity.Microorganisms. 2025 Apr 16;13(4):919. doi: 10.3390/microorganisms13040919. Microorganisms. 2025. PMID: 40284755 Free PMC article.
References
-
- World Health O . Global tuberculosis report 2022. Geneva: World Health Organization; 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

