Crosstalk between DNA methylation and hypoxia in acute myeloid leukaemia
- PMID: 37705055
- PMCID: PMC10500762
- DOI: 10.1186/s13148-023-01566-x
Crosstalk between DNA methylation and hypoxia in acute myeloid leukaemia
Abstract
Background: Acute myeloid leukaemia (AML) is a deadly disease characterised by the uncontrolled proliferation of immature myeloid cells within the bone marrow. Altered regulation of DNA methylation is an important epigenetic driver of AML, where the hypoxic bone marrow microenvironment can help facilitate leukaemogenesis. Thus, interactions between epigenetic regulation and hypoxia signalling will have important implications for AML development and treatment.
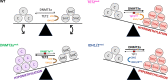
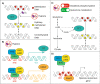
Main body: This review summarises the importance of DNA methylation and the hypoxic bone marrow microenvironment in the development, progression, and treatment of AML. Here, we focus on the role hypoxia plays on signalling and the subsequent regulation of DNA methylation. Hypoxia is likely to influence DNA methylation through altered metabolic pathways, transcriptional control of epigenetic regulators, and direct effects on the enzymatic activity of epigenetic modifiers. DNA methylation may also prevent activation of hypoxia-responsive genes, demonstrating bidirectional crosstalk between epigenetic regulation and the hypoxic microenvironment. Finally, we consider the clinical implications of these interactions, suggesting that reduced cell cycling within the hypoxic bone marrow may decrease the efficacy of hypomethylating agents.
Conclusion: Hypoxia is likely to influence AML progression through complex interactions with DNA methylation, where the therapeutic efficacy of hypomethylating agents may be limited within the hypoxic bone marrow. To achieve optimal outcomes for AML patients, future studies should therefore consider co-treatments that can promote cycling of AML cells within the bone marrow or encourage their dissociation from the bone marrow.
Keywords: Acute myeloid leukaemia; DNA methylation; Epigenetics; Hypoxia; Reactive oxygen species; Treatment outcomes.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Hypoxia impairs decitabine-induced expression of HLA-DR in acute myeloid leukaemia cell lines.Clin Epigenetics. 2025 Jan 17;17(1):8. doi: 10.1186/s13148-025-01812-4. Clin Epigenetics. 2025. PMID: 39825372 Free PMC article.
-
Exploiting epigenetically mediated changes: Acute myeloid leukemia, leukemia stem cells and the bone marrow microenvironment.Adv Cancer Res. 2019;141:213-253. doi: 10.1016/bs.acr.2018.12.005. Epub 2019 Jan 21. Adv Cancer Res. 2019. PMID: 30691684 Review.
-
The Role of Hypoxic Bone Marrow Microenvironment in Acute Myeloid Leukemia and Future Therapeutic Opportunities.Int J Mol Sci. 2021 Jun 25;22(13):6857. doi: 10.3390/ijms22136857. Int J Mol Sci. 2021. PMID: 34202238 Free PMC article. Review.
-
IL-8 as mediator in the microenvironment-leukaemia network in acute myeloid leukaemia.Sci Rep. 2015 Dec 17;5:18411. doi: 10.1038/srep18411. Sci Rep. 2015. PMID: 26674118 Free PMC article.
-
Hypermethylation and down-regulation of DLEU2 in paediatric acute myeloid leukaemia independent of embedded tumour suppressor miR-15a/16-1.Mol Cancer. 2014 May 24;13:123. doi: 10.1186/1476-4598-13-123. Mol Cancer. 2014. PMID: 24885794 Free PMC article.
Cited by
-
Identification of hub genes and potential molecular mechanisms related to drug sensitivity in acute myeloid leukemia based on machine learning.Front Pharmacol. 2024 Apr 8;15:1359832. doi: 10.3389/fphar.2024.1359832. eCollection 2024. Front Pharmacol. 2024. PMID: 38650628 Free PMC article.
-
Relapsed Acute Myeloid Leukemia With Early Presentation As Leukemia Cutis, Refractory to Second-Line Treatment.Cureus. 2025 Mar 5;17(3):e80114. doi: 10.7759/cureus.80114. eCollection 2025 Mar. Cureus. 2025. PMID: 40190958 Free PMC article.
-
Hypoxia impairs decitabine-induced expression of HLA-DR in acute myeloid leukaemia cell lines.Clin Epigenetics. 2025 Jan 17;17(1):8. doi: 10.1186/s13148-025-01812-4. Clin Epigenetics. 2025. PMID: 39825372 Free PMC article.
References
-
- Beckmann K, Kearney AMBJ, Yeung D, Hiwase D, Li M, Roder DM. Changes in five-year survival for people with acute leukaemia in South Australia, 1980–2016. Med J Aust. 2022;216(6):296–302. - PubMed
-
- Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87. - PubMed
-
- Murphy T, Yee KWL. Cytarabine and daunorubicin for the treatment of acute myeloid leukemia. Expert Opin Pharmacother. 2017;18(16):1765–1780. - PubMed
-
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

