Implementing contingency management for stimulant use in opioid treatment programs: protocol of a type III hybrid effectiveness-stepped-wedge trial
- PMID: 37705093
- PMCID: PMC10498624
- DOI: 10.1186/s13012-023-01297-w
Implementing contingency management for stimulant use in opioid treatment programs: protocol of a type III hybrid effectiveness-stepped-wedge trial
Abstract
Background: Contingency management (CM) is an evidence-based intervention for stimulant use and is highly effective in combination with medication for opioid use disorder. Yet, uptake of CM in opioid treatment programs that provide medication for opioid use disorder remains low. This paradox in which CM is one of the most effective interventions, yet one of the least available, represents one of the greatest research-to-practice gaps in the addiction health services field. Multi-level implementation strategies are needed to address barriers to CM implementation at both the provider- and organization-level. This type III hybrid effectiveness-implementation trial was funded by the National Institute on Drug Abuse to evaluate whether a multi-level implementation strategy, the Science of Service Laboratory (SSL), can effectively promote CM implementation in opioid treatment programs. Specific aims will test the effectiveness of the SSL on implementation outcomes (primary aim) and patient outcomes (secondary aim), as well as test putative mediators of implementation outcomes (exploratory aim).
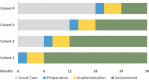
Methods: Utilizing a fully powered type III hybrid effectiveness-implementation trial with a stepped wedge design, we propose to randomize a cohort of 10 opioid treatment programs to receive the SSL across four steps. Each step, an additional 2-3 opioid treatment programs will receive the SSL implementation strategy, which has three core components: didactic training, performance feedback, and external facilitation. At six intervals, each of the 10 opioid treatment programs will provide de-identified electronic medical record data from all available patient charts on CM delivery and patient outcomes. Staff from each opioid treatment program will provide feedback on contextual determinants influencing implementation at three timepoints.
Discussion: Between planning of this protocol and receipt of funding, the landscape for CM in the USA changed dramatically, with multiple Departments of Health launching state-wide CM initiatives. We therefore accelerated the protocol timeline and offered some cursory training resources to all sites as a preparation activity. We also began partnering with multiple Departments of Health to evaluate their rollout of CM using the measures outlined in this protocol.
Trial registration: This study protocol is registered via ClinicalTrials.gov Identifier: NCT05702021. Date of registration: January 27, 2023.
Keywords: Hybrid effectiveness-implementation; Implementation; Stepped wedge trial; Substance use disorders.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Implementing Alcohol Misuse SBIRT in a National Cohort of Pediatric Trauma Centers-a type III hybrid effectiveness-implementation trial.Implement Sci. 2018 Feb 22;13(1):35. doi: 10.1186/s13012-018-0725-x. Implement Sci. 2018. PMID: 29471849 Free PMC article.
-
Implementation facilitation to promote emergency department-initiated buprenorphine for opioid use disorder: protocol for a hybrid type III effectiveness-implementation study (Project ED HEALTH).Implement Sci. 2019 May 7;14(1):48. doi: 10.1186/s13012-019-0891-5. Implement Sci. 2019. PMID: 31064390 Free PMC article.
-
A facilitation model for implementing quality improvement practices to enhance outpatient substance use disorder treatment outcomes: a stepped-wedge randomized controlled trial study protocol.Implement Sci. 2021 Jan 7;16(1):5. doi: 10.1186/s13012-020-01076-x. Implement Sci. 2021. PMID: 33413493 Free PMC article.
-
Stepped implementation-to-target: a study protocol of an adaptive trial to expand access to addiction medications.Implement Sci. 2022 Sep 29;17(1):64. doi: 10.1186/s13012-022-01239-y. Implement Sci. 2022. PMID: 36175963 Free PMC article.
-
Programs for the Reduction or Discontinuation of Opioids or Opioid Substitution Therapy: A Review of the Clinical Effectiveness [Internet].Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2018 Sep 7. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2018 Sep 7. PMID: 30830731 Free Books & Documents. Review.
Cited by
-
Contingency management needs implementation science.Addiction. 2024 Sep;119(9):1522-1524. doi: 10.1111/add.16579. Epub 2024 Jun 16. Addiction. 2024. PMID: 38881151 Free PMC article.
-
Community Impact of Capacity-Building to Develop Trauma Resilient Communities.Adm Policy Ment Health. 2025 May;52(3):506-519. doi: 10.1007/s10488-024-01429-4. Epub 2025 Feb 17. Adm Policy Ment Health. 2025. PMID: 39961916 Free PMC article.
-
Evidence based QUality Improvement for Prescribing Stewardship in ICU (EQUIPS-ICU): protocol for type III hybrid implementation-effectiveness study.Implement Sci. 2025 Feb 25;20(1):12. doi: 10.1186/s13012-024-01413-4. Implement Sci. 2025. PMID: 40001051 Free PMC article.
-
Using the IFASIS (Inventory of Factors Affecting Successful Implementation and Sustainment) to Advance Context-Specific and Generalizable Knowledge of Implementation Determinants: Case Study of a Digital Contingency Management Platform.Res Sq [Preprint]. 2024 Oct 21:rs.3.rs-4912858. doi: 10.21203/rs.3.rs-4912858/v1. Res Sq. 2024. Update in: Implement Sci Commun. 2025 Apr 7;6(1):35. doi: 10.1186/s43058-025-00708-x. PMID: 39502779 Free PMC article. Updated. Preprint.
-
Digitally delivered contingency management during methadone treatment for people with co-occurring cocaine and opioid use: a protocol for a randomized controlled trial.Front Psychiatry. 2025 Jul 2;16:1576277. doi: 10.3389/fpsyt.2025.1576277. eCollection 2025. Front Psychiatry. 2025. PMID: 40673228 Free PMC article.
References
-
- Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: a meta-analysis. Drug Alcohol Depend. 2000;58(1–2):55–66. - PubMed
-
- Resnick RB, Galanter M, Pycha C, Cohen A, Grandison P, Flood N. Buprenorphine: an alternative to methadone for heroin dependence treatment. Psychopharmacol Bull. 1992;28(1):109–113. - PubMed
-
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377(9776):1506–1513. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

