Rotation of the c-Ring Promotes the Curvature Sorting of Monomeric ATP Synthases
- PMID: 37705095
- PMCID: PMC10625105
- DOI: 10.1002/advs.202301606
Rotation of the c-Ring Promotes the Curvature Sorting of Monomeric ATP Synthases
Abstract
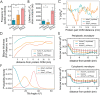
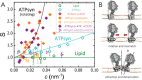
ATP synthases are proteins that catalyse the formation of ATP through the rotatory movement of their membrane-spanning subunit. In mitochondria, ATP synthases are found to arrange as dimers at the high-curved edges of cristae. Here, a direct link is explored between the rotatory movement of ATP synthases and their preference for curved membranes. An active curvature sorting of ATP synthases in lipid nanotubes pulled from giant vesicles is found. Coarse-grained simulations confirm the curvature-seeking behaviour of rotating ATP synthases, promoting reversible and frequent protein-protein contacts. The formation of transient protein dimers relies on the membrane-mediated attractive interaction of the order of 1.5 kB T produced by a hydrophobic mismatch upon protein rotation. Transient dimers are sustained by a conic-like arrangement characterized by a wedge angle of θ ≈ 50°, producing a dynamic coupling between protein shape and membrane curvature. The results suggest a new role of the rotational movement of ATP synthases for their dynamic self-assembly in biological membranes.
Keywords: E. coli; F1Fo ATP synthase; giant vesicles; lipid nanotubes; micromanipulation.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
How rotating ATP synthases can modulate membrane structure.Arch Biochem Biophys. 2021 Sep 15;708:108939. doi: 10.1016/j.abb.2021.108939. Epub 2021 May 28. Arch Biochem Biophys. 2021. PMID: 34052190 Review.
-
Interface mobility between monomers in dimeric bovine ATP synthase participates in the ultrastructure of inner mitochondrial membranes.Proc Natl Acad Sci U S A. 2021 Feb 23;118(8):e2021012118. doi: 10.1073/pnas.2021012118. Proc Natl Acad Sci U S A. 2021. PMID: 33542155 Free PMC article.
-
Dimers of mitochondrial ATP synthase induce membrane curvature and self-assemble into rows.Proc Natl Acad Sci U S A. 2019 Mar 5;116(10):4250-4255. doi: 10.1073/pnas.1816556116. Epub 2019 Feb 13. Proc Natl Acad Sci U S A. 2019. PMID: 30760595 Free PMC article.
-
Mitochondrial permeability transition involves dissociation of F1FO ATP synthase dimers and C-ring conformation.EMBO Rep. 2017 Jul;18(7):1077-1089. doi: 10.15252/embr.201643602. Epub 2017 May 31. EMBO Rep. 2017. PMID: 28566520 Free PMC article.
-
Structure and Mechanisms of F-Type ATP Synthases.Annu Rev Biochem. 2019 Jun 20;88:515-549. doi: 10.1146/annurev-biochem-013118-110903. Epub 2019 Mar 22. Annu Rev Biochem. 2019. PMID: 30901262 Review.
Cited by
-
Origin and evolution of mitochondrial inner membrane composition.J Cell Sci. 2025 May 1;138(9):jcs263780. doi: 10.1242/jcs.263780. Epub 2025 Apr 23. J Cell Sci. 2025. PMID: 40265338 Free PMC article. Review.
-
Cardiolipin acyl chain composition tailors the conformation of mammalian ATP synthase dimers.Commun Chem. 2025 Jul 30;8(1):220. doi: 10.1038/s42004-025-01611-1. Commun Chem. 2025. PMID: 40738953 Free PMC article.
-
Curvature Footprints of Transmembrane Proteins in Simulations with the Martini Force Field.J Phys Chem B. 2024 Jun 27;128(25):5987-5994. doi: 10.1021/acs.jpcb.4c01385. Epub 2024 Jun 11. J Phys Chem B. 2024. PMID: 38860934 Free PMC article.
-
Ablation of Atp5if1 impairs metabolic reprogramming and proliferation of T lymphocytes and compromises mouse survival.iScience. 2024 May 3;27(6):109863. doi: 10.1016/j.isci.2024.109863. eCollection 2024 Jun 21. iScience. 2024. PMID: 38799559 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
