A Mechanically Resilient and Tissue-Conformable Hydrogel with Hemostatic and Antibacterial Capabilities for Wound Care
- PMID: 37705116
- PMCID: PMC10602564
- DOI: 10.1002/advs.202303651
A Mechanically Resilient and Tissue-Conformable Hydrogel with Hemostatic and Antibacterial Capabilities for Wound Care
Abstract
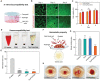
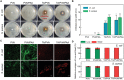
Hydrogels are used in wound dressings because of their tissue-like softness and biocompatibility. However, the clinical translation of hydrogels remains challenging because of their long-term stability, water swellability, and poor tissue adhesiveness. Here, tannic acid (TA) is introduced into a double network (DN) hydrogel consisting of poly(vinyl alcohol) (PVA) and poly(acrylic acid) (PAA) to realize a tough, self-healable, nonswellable, conformally tissue-adhesive, hemostatic, and antibacterial hydrogel. The TA within the DN hydrogel forms a dynamic network, enabling rapid self-healing (within 5 min) and offering effective energy dissipation for toughness and viscoelasticity. Furthermore, the hydrophobic moieties of TA provide a water-shielding effect, rendering the hydrogel nonswellable. A simple chemical modification to the hydrogel further strengthens its interfacial adhesion with tissues (shear strength of ≈31 kPa). Interestingly, the TA also can serve as an effective hemostatic (blood-clotting index of 58.40 ± 1.5) and antibacterial component, which are required for a successful wound dressing. The antibacterial effects of the hydrogel are tested against Escherichia coli and Staphylococcus aureus. Finally, the hydrogel is prepared in patch form and applied to a mouse model to test in vivo biocompatibility and hemostatic performances.
Keywords: antibacterial; hemostasis; hydrogels; tissue adhesives; wound dressing.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Taboada G. M., Yang K., Pereira M. J. N., Liu S. S., Hu Y., Karp J. M., Artzi N., Lee Y., Nat. Rev. Mater. 2020, 5, 310.
-
- Park J., Kim Y., Chun B., Seo J., Biotechnol. J. 2021, 16, 2100231. - PubMed
-
- a) Zhou D., Li S., Pei M., Yang H., Gu S., Tao Y., Ye D., Zhou Y., Xu W., Xiao P., ACS Appl. Mater. Interfaces 2020, 12, 18225; - PubMed
- b) Yang J., Bai R., Chen B., Suo Z., Adv. Funct. Mater. 2020, 30, 1901693.
-
- Yuk H., Lu B., Zhao X., Chem. Soc. Rev. 2019, 48, 1642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
