First Potent Macrocyclic A3 Adenosine Receptor Agonists Reveal G-Protein and β-Arrestin2 Signaling Preferences
- PMID: 37705595
- PMCID: PMC10496144
- DOI: 10.1021/acsptsci.3c00126
First Potent Macrocyclic A3 Adenosine Receptor Agonists Reveal G-Protein and β-Arrestin2 Signaling Preferences
Abstract
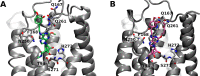
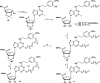
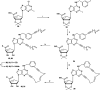
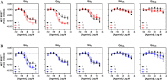
(N)-Methanocarba adenosine derivatives (A3 adenosine receptor (AR) agonists containing bicyclo[3.1.0]hexane replacing furanose) were chain-extended at N6 and C2 positions with terminal alkenes for ring closure. The resulting macrocycles of 17-20 atoms retained affinity, indicating a spatially proximal orientation of these receptor-bound chains, consistent with molecular modeling of 12. C2-Arylethynyl-linked macrocycle 19 was more A3AR-selective than 2-ether-linked macrocycle 12 (both 5'-methylamides, human (h) A3AR affinities (Ki): 22.1 and 25.8 nM, respectively), with lower mouse A3AR affinities. Functional hA3AR comparison of two sets of open/closed analogues in β-arrestin2 and Gi/o protein assays showed certain signaling preferences divergent from reference agonist Cl-IB-MECA 1. The potencies of 1 at all three Gαi isoforms were slightly less than its hA3AR binding affinity (Ki: 1.4 nM), while the Gαi1 and Gαi2 potencies of macrocycle 12 were roughly an order of magnitude higher than its radioligand binding affinity. Gαi2-coupling was enhanced in macrocycle 12 (EC50 2.56 nM, ∼40% greater maximal efficacy than 1). Di-O-allyl precursor 18 cyclized to form 19, increasing the Gαi1 potency by 7.5-fold. The macrocycles 12 and 19 and their open precursors 11 and 18 potently stimulated β-arrestin2 recruitment, with EC50 values (nM) of 5.17, 4.36, 1.30, and 4.35, respectively, and with nearly 50% greater efficacy compared to 1. This example of macrocyclization altering the coupling pathways of small-molecule (nonpeptide) GPCR agonists is the first for potent and selective macrocyclic AR agonists. These initial macrocyclic derivatives can serve as a guide for the future design of macrocyclic AR agonists displaying unanticipated pharmacology.
© 2023 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Jacobson K. A.; Merighi S.; Varani K.; Borea P. A.; Baraldi S.; Aghazadeh Tabrizi M.; Romagnoli R.; Baraldi P. G.; Ciancetta A.; Tosh D. K.; Gao Z. G.; Gessi S. A3 adenosine receptors as modulators of inflammation: from medicinal chemistry to therapy. Med. Res. Rev. 2018, 38, 1031–1072. 10.1002/med.21456. - DOI - PMC - PubMed
-
- Stemmer S. M.; Manojlovic N. S.; Marinca M. V.; Petrov P.; Cherciu N.; Ganea D.; Ciuleanu T. E.; Pusca I. A.; Beg M. S.; Purcell W. T.; Croitoru A. E.; Ilieva R. N.; Natošević S.; Nita A. L.; Kalev D. N.; Harpaz Z.; Farbstein M.; Silverman M. H.; Bristol D.; Itzhak I.; Fishman P. Namodenoson in advanced hepatocellular carcinoma and Child–Pugh B cirrhosis: Randomized placebo-controlled clinical trial. Cancers 2021, 13, 187. 10.3390/cancers13020187. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
