FGF2 Alleviates Microvascular Ischemia-Reperfusion Injury by KLF2-mediated Ferroptosis Inhibition and Antioxidant Responses
- PMID: 37705747
- PMCID: PMC10496511
- DOI: 10.7150/ijbs.85692
FGF2 Alleviates Microvascular Ischemia-Reperfusion Injury by KLF2-mediated Ferroptosis Inhibition and Antioxidant Responses
Abstract
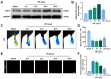
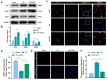
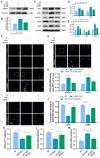
An essential pathogenic element of acute limb ischemia/reperfusion (I/R) injury is microvascular dysfunction. The majority of studies indicates that fibroblast growth factor 2 (FGF2) exhibits protective properties in cases of acute I/R injury. Albeit its specific role in the context of acute limb I/R injury is yet unknown. An impressive post-reperfusion increase in FGF2 expression was seen in a mouse model of hind limb I/R, followed by a decline to baseline levels, suggesting a key role for FGF2 in limb survivability. FGF2 appeared to reduce I/R-induced hypoperfusion, tissue edema, skeletal muscle fiber injury, as well as microvascular endothelial cells (ECs) damage within the limb, according to assessments of limb vitality, Western blotting, and immunofluorescence results. The bioinformatics analysis of RNA-sequencing revealed that ferroptosis played a key role in FGF2-facilitated limb preservation. Pharmacological inhibition of NFE2L2 prevented ECs from being affected by FGF2's anti-oxidative and anti-ferroptosis activities. Additionally, silencing of kruppel-like factor 2 (KLF2) by interfering RNA eliminated the antioxidant and anti-ferroptosis effects of FGF2 on ECs. Further research revealed that the AMPK-HDAC5 signal pathway is the mechanism via which FGF2 regulates KLF2 activity. Data from luciferase assays demonstrated that overexpression of HDAC5 prevented KLF2 from becoming activated by FGF2. Collectively, FGF2 protects microvascular ECs from I/R injury by KLF2-mediated ferroptosis inhibition and antioxidant responses.
Keywords: FGF2; Ferroptosis; Limb ischemia/reperfusion; Microvascular damage; Oxidative stress.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Girn HR, Ahilathirunayagam S, Mavor AI, Homer-Vanniasinkam S. Reperfusion syndrome: cellular mechanisms of microvascular dysfunction and potential therapeutic strategies. Vasc Endovascular Surg. 2007;41:277–93. - PubMed
-
- Kerrigan CL, Stotland MA. Ischemia reperfusion injury: a review. Microsurgery. 1993;14:165–75. - PubMed
-
- McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–63. - PubMed
-
- Wang WZ, Fang XH, Stepheson LL, Khiabani KT, Zamboni WA. Acute microvascular action of vascular endothelial growth factor in skeletal muscle ischemia/reperfusion injury. Plast Reconstr Surg. 2005;115:1355–65. - PubMed
-
- Granger DN, Kvietys PR. Reperfusion therapy-What's with the obstructed, leaky and broken capillaries? Pathophysiology. 2017;24:213–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

