Development of subtype-selective estrogen receptor modulators using the bis(4-hydroxyphenyl)silanol core as a stable isostere of bis(4-hydroxyphenyl)methanol
- PMID: 37705989
- PMCID: PMC10496907
- DOI: 10.1039/d3ra04656g
Development of subtype-selective estrogen receptor modulators using the bis(4-hydroxyphenyl)silanol core as a stable isostere of bis(4-hydroxyphenyl)methanol
Abstract
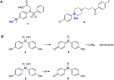
In this study, we synthesized and evaluated silanol-based bisphenol derivatives as stable isosteres of bis(4-hydroxyphenyl)methanol. The developed silanols exhibited estrogen receptor (ER)-modulating activity. Among them, bis(4-hydroxyphenyl)(methyl)silanol (5a) showed a characteristic ER subtype selectivity, namely, antagonistic activity toward ERα and agonistic activity toward ERβ. Docking simulation indicated that the silanol moiety plays a key role in this selectivity. Our results suggest that silanol-based bisphenols offer a unique scaffold for biologically active compounds.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
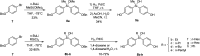



Similar articles
-
Investigations on the effects of basic side chains on the hormonal profile of (4R,5S)/(4S,5R)-4,5-bis(4-hydroxyphenyl)-2-imidazolines.J Med Chem. 2005 Jan 27;48(2):466-74. doi: 10.1021/jm040855c. J Med Chem. 2005. PMID: 15658860
-
Synthesis of an estrogen receptor beta-selective radioligand: 5-[18F]fluoro-(2R,3S)-2,3-bis(4-hydroxyphenyl)pentanenitrile and comparison of in vivo distribution with 16alpha-[18F]fluoro-17beta-estradiol.J Med Chem. 2005 Oct 6;48(20):6366-78. doi: 10.1021/jm050121f. J Med Chem. 2005. PMID: 16190762
-
Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues.J Med Chem. 2001 Nov 22;44(24):4230-51. doi: 10.1021/jm010254a. J Med Chem. 2001. PMID: 11708925
-
Estrogen Receptor β as a Possible Double-Edged Sword Molecule in Breast Cancer: A Mechanism of Alteration of Its Role by Exposure to Endocrine-Disrupting Chemicals.Biol Pharm Bull. 2021;44(11):1594-1597. doi: 10.1248/bpb.b21-00468. Biol Pharm Bull. 2021. PMID: 34719637 Review.
-
Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology.J Steroid Biochem Mol Biol. 2000 Nov 30;74(5):279-85. doi: 10.1016/s0960-0760(00)00104-7. J Steroid Biochem Mol Biol. 2000. PMID: 11162936 Review.
Cited by
-
The role of silicon in drug discovery: a review.RSC Med Chem. 2024 Jul 1;15(10):3286-3344. doi: 10.1039/d4md00169a. eCollection 2024 Oct 17. RSC Med Chem. 2024. PMID: 39430101 Free PMC article. Review.
References
-
- Tacke R. Heinrich T. Bertermann R. Burschka C. Hamacher A. Kassack M. U. Organometallics. 2004;23:4468–4477. doi: 10.1021/om040067l. - DOI
- Tacke R. Popp F. Müller B B. Theis B. Burschka C. Hamacher A. Kassack M. U. Schepmann D. Wünsch B. Jurva U. Wellner E. ChemMedChem. 2008;3:152–164. doi: 10.1002/cmdc.200700205. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

