Potential Mechanism of Fatigue Induction and Its Management by JAK Inhibitors in Inflammatory Rheumatic Diseases
- PMID: 37706062
- PMCID: PMC10497048
- DOI: 10.2147/JIR.S414739
Potential Mechanism of Fatigue Induction and Its Management by JAK Inhibitors in Inflammatory Rheumatic Diseases
Abstract
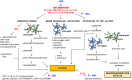
It is well known that fatigue is a highly disabling symptom commonly observed in inflammatory rheumatic diseases (IRDs). Fatigue is strongly associated with a poor quality of life and seems to be an independent predictor of job loss and disability in patients with different rheumatic diseases. Although the pathogenesis of fatigue remains unclear, indirect data suggest the cooperation of the immune system, the central and autonomic nervous system, and the neuroendocrine system in the induction and sustainment of fatigue in chronic diseases. Fatigue does not correspond with disease activity and its mechanism in IRDs. It is suggested that it may change over time and vary between individuals. Abnormal production of pro-inflammatory cytokines such as interleukin-6 (IL-6), interferons (IFNs), granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF, IL-15, IL-17 play a role in both IRDs and subsequent fatigue development. Some of these cytokines such as IL-6, IFNs, GM-CSF, and common gamma-chain cytokines (IL-15, IL-2, and IL-7) activate the Janus Kinases (JAKs) family of intracellular tyrosine kinases. Therapy blocking JAKs (JAK inhibitors - JAKi) has been recently proven to be an effective approach for IRDs treatment, more efficient in pain reduction than anti-TNF. Therefore, the administration of JAKi to IRDs patients experiencing fatigue may find rational implications as a therapeutic modulator not only of disease inflammatory symptoms but also fatigue with its components like pain and neuropsychiatric features as well. In this review, we demonstrate the latest information on the mechanisms of fatigue in rheumatic diseases and the potential effect of JAKi on fatigue reduction.
Keywords: JAK inhibitors; baricitinib; fatigue; patient-reported outcomes; tofacitinib; upadacitinib.
© 2023 Felis-Giemza et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- Norheim KB, Jonsson G, Omdal R. Biological mechanisms of chronic fatigue. Rheumatology. 2011;50(6):1009–1018. - PubMed
Publication types
LinkOut - more resources
Full Text Sources