High maternal adiposity during pregnancy programs an imbalance in the lipidome and predisposes to diet-induced hepatosteatosis in the offspring
- PMID: 37706282
- PMCID: PMC10550783
- DOI: 10.1042/BSR20231060
High maternal adiposity during pregnancy programs an imbalance in the lipidome and predisposes to diet-induced hepatosteatosis in the offspring
Abstract
Background: Exposure to high maternal adiposity in utero is a significant risk factor for the later-life development of metabolic syndrome (MetS), including non-alcoholic fatty liver disease (NAFLD). We have previously shown that high pre-pregnancy adiposity programs adipose tissue dysfunction in the offspring, leading to spillover of fatty acids into the circulation, a key pathogenic event in obesity-associated MetS. Herein, we hypothesized that programming of adipose tissue dysfunction in offspring born to overweight dams increases the risk for developing NAFLD.
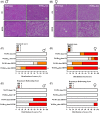
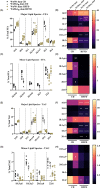
Results: Females heterozygous for leptin receptor deficiency (Hetdb) were used as a model of high pre-pregnancy adiposity. Female wild-type (Wt) offspring born to Hetdb pregnancies gained significantly more body fat following high-fat/fructose diet (HFFD) compared with Wt offspring born to Wt dams. HFFD increased circulating free fatty acids (FFA) in male offspring of control dams, while FFA levels were similar in HFFD-fed offspring from Wt dams and CD or HFFD-fed Wt offspring from Hetdb dams. Despite female-specific protection from diet-induced FFA spillover, both male and female offspring from Hetdb dams were more susceptible to diet-induced hepatosteatosis. Lipidomic analysis revealed that CD-offspring of overweight dams had decreased hepatic polyunsaturated FA (PUFA) levels compared with control offspring. Changes to saturated FA (SFA) and the de novo lipogenic (DNL) index were diet driven; however, there was a significant effect of the intrauterine environment on FA elongation and Δ9 desaturase activity.
Conclusion: High maternal adiposity during pregnancy programs a susceptibility to diet-induced hepatosteatosis.
Keywords: Developmental Programming; Lipidomics; Maternal Obesity; NAFLD.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures




References
-
- Abeysekera K.W., Orr J.G., Madley-Dowd P., Fernandes G.S., Zuccolo L., Gordon F.H.et al. . (2021) Association of maternal pre-pregnancy BMI and breastfeeding with NAFLD in young adults: a parental negative control study. Lancet Reg. Health - Eur. 10, 100206 10.1016/j.lanepe.2021.100206 - DOI - PMC - PubMed
-
- Mikolajczak A., Sallam N.A., Singh R.D., Scheidl T.B., Walsh E.J., Larion S.et al. . (2021) Accelerated developmental adipogenesis programs adipose tissue dysfunction and cardiometabolic risk in offspring born to dams with metabolic dysfunction. Am. J. Physiol.-Endocrinol. Metab. 321, E581–E591 10.1152/ajpendo.00229.2021 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

