Combined Targeting of NAD Biosynthesis and the NAD-dependent Transcription Factor C-terminal Binding Protein as a Promising Novel Therapy for Pancreatic Cancer
- PMID: 37707363
- PMCID: PMC10549224
- DOI: 10.1158/2767-9764.CRC-22-0521
Combined Targeting of NAD Biosynthesis and the NAD-dependent Transcription Factor C-terminal Binding Protein as a Promising Novel Therapy for Pancreatic Cancer
Abstract
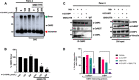
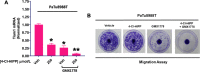
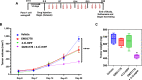
Cancer therapies targeting metabolic derangements unique to cancer cells are emerging as a key strategy to address refractory solid tumors such as pancreatic ductal adenocarcinomas (PDAC) that exhibit resistance to extreme nutrient deprivation in the tumor microenvironment. Nicotinamide adenine dinucleotide (NAD) participates in multiple metabolic pathways and nicotinamide phosphoribosyl transferase (NAMPT) is one of the key intracellular enzymes that facilitate the synthesis of NAD. C-terminal binding proteins 1 and 2 (CtBP) are paralogous NAD-dependent oncogenic transcription factors and dehydrogenases that nucleate an epigenetic complex regulating a cohort of genes responsible for cancer proliferation and metastasis. As adequate intracellular NAD is required for CtBP to oligomerize and execute its oncogenic transcriptional coregulatory activities, we hypothesized that NAD depletion would synergize with CtBP inhibition, improving cell inhibitory efficacy. Indeed, depletion of cellular NAD via the NAMPT inhibitor GMX1778 enhanced growth inhibition induced by either RNAi-mediated CtBP1/2 knockdown or the CtBP dehydrogenase inhibitor 4-chlorophenyl-2-hydroxyimino propanoic acid as much as 10-fold in PDAC cells, while untransformed pancreatic ductal cells were unaffected. The growth inhibitory effects of the NAMPT/CtBP inhibitor combination correlated pharmacodynamically with on-target disruption of CtBP1/2 dimerization, CtBP2 interaction with the CoREST epigenetic regulator, and transcriptional activation of the oncogenic target gene TIAM1. Moreover, this same therapeutic combination strongly attenuated growth of PDAC cell line xenografts in immunodeficient mice, with no observable toxicity. Collectively, our data demonstrate that targeting CtBP in combination with NAD depletion represents a promising therapeutic strategy for PDAC.
Significance: Effective precision therapies are lacking in PDAC. We demonstrate that simultaneous inhibition of NAD metabolism and the oncoprotein CtBP is potently effective at blocking growth of both PDAC cells in culture and human PDAC-derived tumors in mice and should be explored further as a potential therapy for patients with PDAC.
© 2023 The Authors; Published by the American Association for Cancer Research.
Figures


References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. - PubMed
-
- Olivares O, Vasseur S. Metabolic rewiring of pancreatic ductal adenocarcinoma: new routes to follow within the maze. Int J Cancer 2016;138:787–96. - PubMed
-
- Demarest TG, Babbar M, Okur MN, Dan X, Croteau DL, Fakouri NB, et al. . NAD+ metabolism in aging and cancer. Annu Rev Cancer Biol 2019;3:105–30.
-
- Sampath D, Zabka TS, Misner DL, O'Brien T, Dragovich PS. Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) as a therapeutic strategy in cancer. Pharmacol Ther 2015;151:16–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

