Electroresponse of weak polyelectrolyte brushes
- PMID: 37707751
- PMCID: PMC10501941
- DOI: 10.1140/epje/s10189-023-00341-3
Electroresponse of weak polyelectrolyte brushes
Abstract
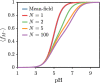
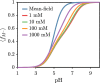
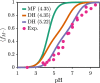
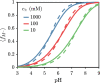
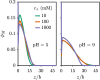
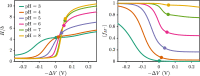
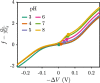
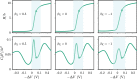
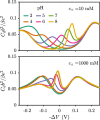
End-tethered polyelectrolytes are widely used to modify substrate properties, particularly for lubrication or wetting. External stimuli, such as pH, salt concentration, or an electric field, can induce profound structural responses in weak polyelectrolyte brushes, which can be utilized to further tune substrate properties. We study the structure and electroresponsiveness of weak polyacid brushes using an inhomogeneous theory that incorporates both electrostatic and chain connectivity correlations at the Debye-Hückel level. Our calculation shows that a weak polyacid brush swells under the application of a negative applied potential, in agreement with recent experimental observation. We rationalize this behavior using a scaling argument that accounts for the effect of the surface charge. We also show that the swelling behavior has a direct influence on the differential capacitance, which can be modulated by the solvent quality, pH, and salt concentration.
© 2023. The Author(s).
Figures

References
-
- Ali M, Yameen B, Neumann R, Ensinger W, Knoll W, Azzaroni O. Biosensing and supramolecular bioconjugation in single conical polymer nanochannels. Facile incorporation of biorecognition elements into nanoconfined geometries. J. Am. Chem. Soc. 2008;130(48):16351–16357. doi: 10.1021/ja8071258. - DOI - PubMed
-
- Ma S, Ye Q, Pei X, Wang D, Zhou F. Antifouling on Gecko’s feet inspired fibrillar surfaces: evolving from land to marine and from liquid repellency to algae resistance. Adv. Mater. Interfaces. 2015;2(13):1500257. doi: 10.1002/admi.201500257. - DOI
-
- Higaki Y, Kobayashi M, Murakami D, Takahara A. Anti-fouling behavior of polymer brush immobilized surfaces. Polym. J. 2016;48(4):325–331. doi: 10.1038/pj.2015.137. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

