A Model for Empowering Rural Solutions for Cervical Cancer Prevention (He Tapu Te Whare Tangata): Protocol for a Cluster Randomized Crossover Trial
- PMID: 37707939
- PMCID: PMC10540018
- DOI: 10.2196/51643
A Model for Empowering Rural Solutions for Cervical Cancer Prevention (He Tapu Te Whare Tangata): Protocol for a Cluster Randomized Crossover Trial
Abstract
Background: Māori are the Indigenous people of Aotearoa (New Zealand). Despite global acceptance that cervical cancer is almost entirely preventable through vaccination and screening, wāhine Māori (Māori women) are more likely to have cervical cancer and 2.5 times more likely to die from it than non-Māori women. Rural Māori residents diagnosed with cervical cancer have worse outcomes than urban residents. Living in rural Aotearoa means experiencing barriers to appropriate and timely health care, resulting from distance, the lack of community resourcing, and low prioritization of rural needs by the health system and government. These barriers are compounded by the current screening processes and referral pathways that create delays at each step. Screening for high-risk human papillomavirus (hrHPV) and point-of-care (POC) testing are scientific advances used globally to prevent cervical cancer.
Objective: This study aims to compare acceptability, feasibility, timeliness, referral to, and attendance for colposcopy following hrHPV detection between a community-controlled pathway and standard care.
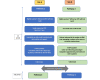
Methods: This is a cluster randomized crossover trial, with 2 primary care practices (study sites) as clusters. Each site was randomized to implement either pathway 1 or 2, with crossover occurring at 15 months. Pathway 1 (community-controlled pathway) comprises HPV self-testing, 1-hour POC results, face-to-face information, support, and immediate referral to colposcopy for women with a positive test result. Pathway 2 (standard care) comprises HPV self-testing, laboratory analysis, usual results giving, information, support, and standard referral pathways for women with a positive test result. The primary outcome is the proportion of women with hrHPV-positive results having a colposcopy within 20 working days of the HPV test (national performance indicator). Qualitative research will analyze successes and challenges of both pathways from the perspectives of governance groups, clinical staff, women, and their family. This information will directly inform the new National Cervical Screening Program.
Results: In the first 15-month period, 743 eligible HPV self-tests were performed: 370 in pathway 1 with POC testing and 373 in pathway 2 with laboratory testing. The positivity rate for hrHPV was 7.3% (54/743). Data collection for the second period, qualitative interviews, and analyses are ongoing.
Conclusions: This Māori-centered study combines quantitative and qualitative research to compare 2 clinical pathways from detection of hrHPV to colposcopy. This protocol draws on rural community practices strengths, successfully engaging Māori from a whānau ora (family wellness) approach including kanohi ki te kanohi (face-to-face), kaiāwhina (nonclinical community health workers), and multiple venues for interventions. It will inform the theory and practice of rural models of the use of innovative technology, addressing Māori cervical cancer inequities and facilitating Māori wellness. The findings are anticipated to be applicable to other Indigenous and rural people in high-income countries.
Trial registration: Australian New Zealand Clinical Trials Registry (ANZCTR) ACTRN12621000553875; https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12621000553875.
International registered report identifier (irrid): DERR1-10.2196/51643.
Keywords: Indigenous people; Māori; New Zealand; cervical intraepithelial neoplasia; colposcopy; early detection of cancer; health equity; papillomavirus infections; point-of-care systems; primary health care; self-testing; uterine cervical neoplasms.
©Beverley Lawton, Evelyn Jane MacDonald, Francesca Storey, Jo-Ann Stanton, Anna Adcock, Melanie Gibson, Varsha Parag, Ngaire Kereru Sparkes, Bobby Kaimoana, Frances King, Marion Terry, Huti Watson, Matthew Bennett, Charles Seymour Lambert, Stacie Geller, Isitokia Paasi, Merilyn Hibma, Peter Sykes, David Hawkes, Marion Saville. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 14.09.2023.
Conflict of interest statement
Conflicts of Interest: MS is an investigator on the Compass trial for which her organization, The Australian Centre for the Prevention of Cervical Cancer (ACPCC), has received kits and partial funding from Roche for the Compass trial. VCS Pathology has received funding from Copan for independent assessment of Copan products. VCS Pathology has also received Equipment or supplies from Abbott, AusDiagnostics, BD, Cepheid, Copan, Hologic, Microbiologics, MicroBix, NRL, Qiagen, Rovers, Roche, and Seegene for research purposes.
Figures
References
-
- Adcock A, Stevenson K, Cram F, MacDonald EJ, Geller S, Hermens J, Lawton B. He Tapu Te Whare Tangata (sacred house of humanity): under-screened Māori women talk about HPV self-testing cervical screening clinical pathways. Int J Gynaecol Obstet. 2021 Nov 21;155(2):275–81. doi: 10.1002/ijgo.13873. - DOI - PubMed
-
- Harris R, Cormack D, Tobias M, Yeh L-C, Talamaivao N, Minster J, Timutimu R. The pervasive effects of racism: experiences of racial discrimination in New Zealand over time and associations with multiple health domains. Soc Sci Med. 2012 Feb;74(3):408–15. doi: 10.1016/j.socscimed.2011.11.004.S0277-9536(11)00714-3 - DOI - PubMed
-
- Crengle S, Davie G, Whitehead J, de Graaf B, Lawrenson R, Nixon G. Mortality outcomes and inequities experienced by rural Māori in Aotearoa New Zealand. Lancet Reg Health West Pac. 2022 Nov;28:100570. doi: 10.1016/j.lanwpc.2022.100570. https://linkinghub.elsevier.com/retrieve/pii/S2666-6065(22)00185-7 S2666-6065(22)00185-7 - DOI - PMC - PubMed
-
- Cancer. Ministry of Health New Zealand Government. [2023-02-20]. https://www.health.govt.nz/our-work/populations/maori-health/tatau-kahuk... .
LinkOut - more resources
Full Text Sources