The changing characteristics of a cohort of children and adolescents living with HIV at antiretroviral therapy initiation in Asia
- PMID: 37708128
- PMCID: PMC10501581
- DOI: 10.1371/journal.pone.0291523
The changing characteristics of a cohort of children and adolescents living with HIV at antiretroviral therapy initiation in Asia
Abstract
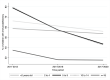
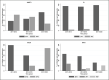
Despite improvements in HIV testing and earlier antiretroviral therapy (ART) initiation in children living with HIV through the years, a considerable proportion start treatment with advanced disease. We studied characteristics of children and adolescents living with HIV and their level of immunodeficiency at ART initiation using data from a multi-country Asian cohort. We included children and adolescents who were ART-naïve and <18 years of age at ART initiation from 2011 to 2020 at 17 HIV clinics in six countries. Incidence rates of opportunistic infections (OIs) in the first two years of triple-drug ART (≥3 antiretrovirals) was also reported. Competing risk regression analysis was performed to identify factors associated with first occurrence of OI. In 2,027 children and adolescents (54% males), median age at ART initiation increased from 4.5 years in 2011-2013 to 6.7 in 2017-2020, median CD4 count doubled from 237 cells/μl to 466 cells/μl, and proportion of children who initiated ART as severely immunodeficient decreased from 70% to 45%. During follow-up, 275 (14%) children who received triple-drug ART as first treatment and had at least one clinic visit, developed at least one OI in the first two years of treatment (9.40 per 100 person-years). The incidence rate of any first OI declined from 12.52 to 7.58 per 100 person-years during 2011-2013 and 2017-2020. Lower hazard of OIs were found in those with age at first ART 2-14 years, current CD4 ≥200 cells/μl, and receiving ART between 2017 and 2020. The analysis demonstrated increasing number of children and adolescents starting ART with high CD4 count at ART start. The rate of first OI markedly decreased in children who started ART in more recent years. There remains a clear need for improvement in HIV control strategies in children, by promoting earlier diagnosis and timely treatment.
Copyright: © 2023 Sornillo et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: AHS receives grants to her institution from ViiV Healthcare. All other authors report no potential conflicts of interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- UNICEF. Global and regional trends [Internet]. 2023. [cited 2023 Aug 18]. Available from: https://data.unicef.org/topic/hivaids/global-regional-trends/
-
- UNAIDS. Global HIV statistics—Fact sheet 2023. [Internet]. [cited 2023 Aug 18]. Available from: https://www.unaids.org/en/resources/fact-sheet
-
- UNICEF. Adolescent HIV treatment [Internet]. 2023. [cited 2023 Aug 18]. Available from: https://data.unicef.org/topic/hivaids/adolescent-hiv-treatment/
-
- Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, et al.. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. 2013/11/12 ed. 2013. Nov 9;382(9904):1555–63. doi: 10.1016/S0140-6736(13)61409-9 - DOI - PMC - PubMed
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. [Internet]. 2016. [cited 2021 Jun 10]. Available from: http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

