The emerging roles of MARCH8 in viral infections: A double-edged Sword
- PMID: 37708148
- PMCID: PMC10501654
- DOI: 10.1371/journal.ppat.1011619
The emerging roles of MARCH8 in viral infections: A double-edged Sword
Abstract
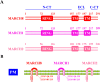
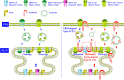
The host cell membrane-associated RING-CH 8 protein (MARCH8), a member of the E3 ubiquitin ligase family, regulates intracellular turnover of many transmembrane proteins and shows potent antiviral activities. Generally, 2 antiviral modes are performed by MARCH8. On the one hand, MARCH8 catalyzes viral envelope glycoproteins (VEGs) ubiquitination and thus leads to their intracellular degradation, which is the cytoplasmic tail (CT)-dependent (CTD) mode. On the other hand, MARCH8 traps VEGs at some intracellular compartments (such as the trans-Golgi network, TGN) but without inducing their degradation, which is the cytoplasmic tail-independent (CTI) mode, by which MARCH8 hijacks furin, a cellular proprotein convertase, to block VEGs cleavage. In addition, the MARCH8 C-terminal tyrosine-based motif (TBM) 222YxxL225 also plays a key role in its CTI antiviral effects. In contrast to its antiviral potency, MARCH8 is occasionally hijacked by some viruses and bacteria to enhance their invasion, indicating a duplex role of MARCH8 in host pathogenic infections. This review summarizes MARCH8's antiviral roles and how viruses evade its restriction, shedding light on novel antiviral therapeutic avenues.
Copyright: © 2023 Yu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
MARCH8 Targets Cytoplasmic Lysine Residues of Various Viral Envelope Glycoproteins.Microbiol Spectr. 2022 Feb 23;10(1):e0061821. doi: 10.1128/spectrum.00618-21. Epub 2022 Jan 12. Microbiol Spectr. 2022. PMID: 35019698 Free PMC article.
-
Mechanism of Viral Glycoprotein Targeting by Membrane-Associated RING-CH Proteins.mBio. 2021 Mar 16;12(2):e00219-21. doi: 10.1128/mBio.00219-21. mBio. 2021. PMID: 33727347 Free PMC article.
-
MARCH8 Inhibits Ebola Virus Glycoprotein, Human Immunodeficiency Virus Type 1 Envelope Glycoprotein, and Avian Influenza Virus H5N1 Hemagglutinin Maturation.mBio. 2020 Sep 15;11(5):e01882-20. doi: 10.1128/mBio.01882-20. mBio. 2020. PMID: 32934085 Free PMC article.
-
MARCH8: the tie that binds to viruses.FEBS J. 2022 Jul;289(13):3642-3654. doi: 10.1111/febs.16017. Epub 2021 May 31. FEBS J. 2022. PMID: 33993615 Review.
-
[Identification of an antiviral host transmembrane protein MARCH8].Uirusu. 2015;65(2):173-178. doi: 10.2222/jsv.65.173. Uirusu. 2015. PMID: 27760915 Review. Japanese.
Cited by
-
Editorial: Unveiling host-pathogen interactions: insights into animal cellular immunity and novel diagnostics.Front Cell Infect Microbiol. 2025 Jan 15;14:1543349. doi: 10.3389/fcimb.2024.1543349. eCollection 2024. Front Cell Infect Microbiol. 2025. PMID: 39882345 Free PMC article. No abstract available.
-
Further Characterization of the Antiviral Transmembrane Protein MARCH8.Cells. 2024 Apr 17;13(8):698. doi: 10.3390/cells13080698. Cells. 2024. PMID: 38667313 Free PMC article.
-
Siah2- and LRSAM1-mediated K63-linked ubiquitination of snakehead vesiculovirus nucleoprotein facilitates viral replication.J Virol. 2024 Jul 23;98(7):e0020224. doi: 10.1128/jvi.00202-24. Epub 2024 Jun 6. J Virol. 2024. PMID: 38842318 Free PMC article.
-
The membrane-associated ubiquitin ligase MARCHF8 stabilizes the human papillomavirus oncoprotein E7 by degrading CUL1 and UBE2L3 in head and neck cancer.J Virol. 2024 Feb 20;98(2):e0172623. doi: 10.1128/jvi.01726-23. Epub 2024 Jan 16. J Virol. 2024. PMID: 38226814 Free PMC article.
-
MARCH8 promotes the proteasomal degradation of foot-and-mouth disease virus VP1, VP2, and VP3 to negatively regulate viral replication.Vet Res. 2025 Apr 30;56(1):96. doi: 10.1186/s13567-025-01521-z. Vet Res. 2025. PMID: 40307900 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

