Identification of genes required for Plasmodium gametocyte-to-sporozoite development in the mosquito vector
- PMID: 37708854
- PMCID: PMC7618085
- DOI: 10.1016/j.chom.2023.08.010
Identification of genes required for Plasmodium gametocyte-to-sporozoite development in the mosquito vector
Abstract
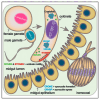
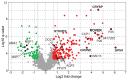
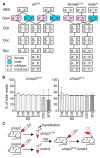
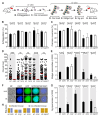
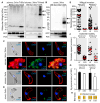
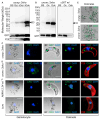
Malaria remains one of the most devastating infectious diseases. Reverse genetic screens offer a powerful approach to identify genes and molecular processes governing malaria parasite biology. However, the complex regulation of gene expression and genotype-phenotype associations in the mosquito vector, along with sexual reproduction, have hindered the development of screens in this critical part of the parasite life cycle. To address this, we developed a genetic approach in the rodent parasite Plasmodium berghei that, in combination with barcode sequencing, circumvents the fertilization roadblock and enables screening for gametocyte-expressed genes required for parasite infection of the mosquito Anopheles coluzzii. Our results confirm previous findings, validating our approach for scaling up, and identify genes necessary for mosquito midgut infection, oocyst development, and salivary gland infection. These findings can aid efforts to study malaria transmission biology and to develop interventions for controlling disease transmission.
Keywords: crystalloid; gametocyte expression; host-parasite interactions; malaria transmission; oocyst; ookinete gliding motility; ookinete vesicle trafficking; salivary gland invasion; signature-tagged mutagenesis; sporozoite infectivity.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Akinosoglou KA, Bushell ES, Ukegbu CV, Schlegelmilch T, Cho JS, Redmond S, Sala K, Christophides GK, Vlachou D. Characterization of Plasmodium developmental transcriptomes in Anopheles gambiae midgut reveals novel regulators of malaria transmission. Cell Microbiol. 2015;17:254–268. doi: 10.1111/cmi.12363. - DOI - PMC - PubMed
-
- Modrzynska K, Pfander C, Chappell L, Yu L, Suarez C, Dundas K, Gomes AR, Goulding D, Rayner JC, Choudhary J, et al. A knockout screen of ApiAP2 genes reveals networks of interacting transcriptional regulators controlling the plasmodium life cycle. Cell Host Microbe. 2017;21:11–22. doi: 10.1016/j.chom.2016.12.003. - DOI - PMC - PubMed
-
- Yuda M, Iwanaga S, Shigenobu S, Mair GR, Janse CJ, Waters AP, Kato T, Kaneko I. Identification of a transcription factor in the mosquito-invasive stage of malaria parasites. Mol Microbiol. 2009;71:1402–1414. - PubMed
-
- Hall N, Karras M, Raine JD, Carlton JM, Kooij TW, Berriman M, Florens L, Janssen CS, Pain A, Christophides GK, et al. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307:82–86. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

