Life expectancy associated with different ages at diagnosis of type 2 diabetes in high-income countries: 23 million person-years of observation
- PMID: 37708900
- PMCID: PMC7615299
- DOI: 10.1016/S2213-8587(23)00223-1
Life expectancy associated with different ages at diagnosis of type 2 diabetes in high-income countries: 23 million person-years of observation
Abstract
Background: The prevalence of type 2 diabetes is increasing rapidly, particularly among younger age groups. Estimates suggest that people with diabetes die, on average, 6 years earlier than people without diabetes. We aimed to provide reliable estimates of the associations between age at diagnosis of diabetes and all-cause mortality, cause-specific mortality, and reductions in life expectancy.
Methods: For this observational study, we conducted a combined analysis of individual-participant data from 19 high-income countries using two large-scale data sources: the Emerging Risk Factors Collaboration (96 cohorts, median baseline years 1961-2007, median latest follow-up years 1980-2013) and the UK Biobank (median baseline year 2006, median latest follow-up year 2020). We calculated age-adjusted and sex-adjusted hazard ratios (HRs) for all-cause mortality according to age at diagnosis of diabetes using data from 1 515 718 participants, in whom deaths were recorded during 23·1 million person-years of follow-up. We estimated cumulative survival by applying age-specific HRs to age-specific death rates from 2015 for the USA and the EU.
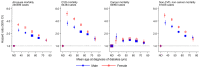
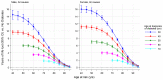
Findings: For participants with diabetes, we observed a linear dose-response association between earlier age at diagnosis and higher risk of all-cause mortality compared with participants without diabetes. HRs were 2·69 (95% CI 2·43-2·97) when diagnosed at 30-39 years, 2·26 (2·08-2·45) at 40-49 years, 1·84 (1·72-1·97) at 50-59 years, 1·57 (1·47-1·67) at 60-69 years, and 1·39 (1·29-1·51) at 70 years and older. HRs per decade of earlier diagnosis were similar for men and women. Using death rates from the USA, a 50-year-old individual with diabetes died on average 14 years earlier when diagnosed aged 30 years, 10 years earlier when diagnosed aged 40 years, or 6 years earlier when diagnosed aged 50 years than an individual without diabetes. Using EU death rates, the corresponding estimates were 13, 9, or 5 years earlier.
Interpretation: Every decade of earlier diagnosis of diabetes was associated with about 3-4 years of lower life expectancy, highlighting the need to develop and implement interventions that prevent or delay the onset of diabetes and to intensify the treatment of risk factors among young adults diagnosed with diabetes.
Funding: British Heart Foundation, Medical Research Council, National Institute for Health and Care Research, and Health Data Research UK.
Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.
Conflict of interest statement
Declaration of interests ASB reports grants outside of this work from AstraZeneca, Bayer, Biogen, BioMarin, Merck, Novartis, and Sanofi. BGN reports consulting fees form AstraZeneca, Sanofi, Regeneron Pharmaceuticals, Ionis Pharmaceuticals, Amgen, Kowa Pharmaceuticals, Denka, Amarin, Novartis, Novo Nordisk, Esperion Therapeutics, Silence Therapeutics, and Ultragenyx; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Sanofi, Amgen, Kowa Pharmaceuticals, Denka, Amarin, Novartis, Novo Nordisk, Abbott Laboratories, Mankind Pharma; and participation on a data safety monitoring board or advisory board for AstraZeneca, Ionis Pharmaceuticals, Kowa Pharmaceuticals, Novartis, and Esperion. BBY reports grants from the National Health and Medical Research Council paid to his institutions (Medical School, The University of Western Australia, Perth, WA, Australia; and Department of Endocrinology and Diabetes, Fiona Stanley Hospital, Perth, WA, Australia). CK report grants from the US National Institutes of Health (NIH); grants or contracts from Grifols and Diagnostica Stago; consulting fees from BMS Pfizer; participation on a data safety monitoring board or advisory board for BMS Pfizer; a leadership or fiduciary role on the RECOVER-CDC Steering Committee; and stock or stock options for Insera. CC reports consulting fees from the Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda, and UCB. DAL reports grants from the UK Medical Research Council (MRC), National Institute for Health and Care Research (NIHR), BHF, European Research Council, NIH, and Diabetes UK paid to her institutions (MRC Integrative Epidemiology Unit and Population Health Science, Bristol Medical School, University of Bristol, Bristol, UK); and participation on a data safety monitoring board or advisory board for the UK Biobank, Bradford Institute Health Research, and NIHR-BHF. DN reports grants outside of this work from GSK and participation on a data safety monitoring board or advisory board for GSK. EDA reports grants from the BHF, NIHR, and an NIHR Senior Investigator Award; and participation on a data safety monitoring board or advisory board from Our Future Health and EURAC Research. HMK reports grants outside of this work from the NIH Agency for Healthcare Research and Quality, Foundation for a Smoke-Free World, State of CT Department of Public Health, US Food and Drug Administration, Johnson & Johnson, American Heart Association, Centers for Medicare & Medicaid Services, Google, and Pfizer; consultant fees from Massachusetts Medical Society, Eyedentifeye, and F-Prime; participation on a data safety monitoring board or advisory board for Aetna, Reality Labs, Element Science, and United Health; stock or stock options for Element Science and Eyedentifeye; and is a co-founder of Hugo Health and Refactor Health. JSu reports stock or stock options from Anagram Kommunikation, and Symptoms Europe. JD reports support from a BHF Professorship and NIHR Senior Investigator Award; and grants or contracts from Merck Sharp & Dohme, Novartis, Pfizer, and AstraZeneca, outside the submitted work. JG report grants or contracts from the MRC; payment or honoraria for lectures and support for attending meetings and/or travel from Yonsei University; and leadership or a fiduciary role for Dementias Platform UK and the BrainWaves study. JSh reports grants or contracts from AstraZeneca; consulting fees from AstraZeneca, Sanofi, Novo Nordisk, MSD, Eli Lilly, and Pfizer; and payment or honoraria for lectures and support for attending meetings and/or travel from Astra Zeneca, Mylan, Sanofi, Boehringer Ingelheim, Zuellig Pharma, and Abbott. KJM reports grants from the NIH. LS is now a full-time employee at Regeneron Genetics Center. LEW reports grants from the National Heart, Lung, and Blood Institute (NHLBI), NIH. NS reports grants paid to their institution (University of Glasgow, Glasgow, UK) from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics; consulting fees from Abbott Laboratories, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Merck, Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, and Sanofi; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Abbott Laboratories, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, and Novo Nordisk. PJN reports grants from NIH. PMR has received institutional research grant support from Kowa Pharmaceuticals, Novartis, Amarin, Pfizer, Esperion, Novo Nordisk, and the NHLBI; has served as a consultant to Novartis, AstraZeneca, Kowa Pharmaceuticals, and Novo Nordisk; and receives compensation for service on the Peter Munk Advisory Board (University of Toronto, Toronto, ON, Canada), the Leducq Foundation, and the Baim Institute (Boston, MA, USA). RTdJ reports grants from Takeda Pharmaceuticals and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Amgen. SGT has received institutional research grant support from the BHF. SJG is a trustee of the Novo Nordisk UK Research Foundation medical research charity. SKi reports grants or contracts from VASCage (Research Centre on Vascular Ageing and Stroke, project number 868624) of the Austrian Research Promotion Agency FFG (COMET program–Competence Centers for Excellent Technologies) funded by the Federal Ministry for Climate Protection, Environment, Energy, Transport, Innovation and Technology; the Federal Ministry for Labour and Economy; and the federal states Tyrol (via Standortagentur), Salzburg, and Vienna (via Vienna Business Agency). SK has received institutional research grant support from the BHF, the UK MRC, the UK NIHR, and Health Data Research UK. SB has received institutional research grant support from the Wellcome Trust and the MRC. SWS reports receiving institutional grants from the NIH, royalties from Springer Publishing, and consulting fees from Fred Hutchinson Cancer Institute and State University of NY at Buffalo. TS reports consulting fees from Amarin, Amgen, Novartis, Orion Pharma, Raisio Group, and Sankyo; and a leadership or fiduciary role for the Finnish guidelines for dyslipidaemia. VS reports receiving institutional grants from Juho Vainio Foundation and Bayer. All other authors declare no competing interests.
Figures
Comment in
-
Many years of life lost to young-onset type 2 diabetes.Lancet Diabetes Endocrinol. 2023 Oct;11(10):709-710. doi: 10.1016/S2213-8587(23)00255-3. Epub 2023 Sep 11. Lancet Diabetes Endocrinol. 2023. PMID: 37708899 No abstract available.
References
-
- Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40. - PubMed
-
- Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA. 2014;312(12):1218–26. - PubMed
-
- International Diabetes Federation. IDF Diabetes Atlas. 10th. International Diabetes Federation; Brussels: [accessed July, 20223]. https://diabetesatlas.orgl .
Publication types
MeSH terms
Grants and funding
- RE/18/1/34212/BHF_/British Heart Foundation/United Kingdom
- RG/19/4/34452/BHF_/British Heart Foundation/United Kingdom
- RG/18/13/33946/BHF_/British Heart Foundation/United Kingdom
- MR/L003120/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00002/7/MRC_/Medical Research Council/United Kingdom
- CH/12/2/29428/BHF_/British Heart Foundation/United Kingdom
- BCDSA\100005/BHF_/British Heart Foundation/United Kingdom
- MC_UU_00006/1/MRC_/Medical Research Council/United Kingdom
- NIHR203312/DH_/Department of Health/United Kingdom
- BRC-1215-20014/DH_/Department of Health/United Kingdom
- MR/R024227/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00032/1/MRC_/Medical Research Council/United Kingdom
- 204623/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- MC_UU_00006/6/MRC_/Medical Research Council/United Kingdom
- 100114/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

