Isoxanthohumol improves obesity and glucose metabolism via inhibiting intestinal lipid absorption with a bloom of Akkermansia muciniphila in mice
- PMID: 37709134
- PMCID: PMC10539672
- DOI: 10.1016/j.molmet.2023.101797
Isoxanthohumol improves obesity and glucose metabolism via inhibiting intestinal lipid absorption with a bloom of Akkermansia muciniphila in mice
Abstract
Objective: Polyphenols have health-promoting effects, such as improving insulin resistance. Isoxanthohumol (IX), a prenylated flavonoid found in beer hops, has been suggested to reduce obesity and insulin resistance; however, the mechanism remains unknown.
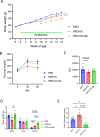
Methods: High-fat diet-fed mice were administered IX. We analyzed glucose metabolism, gene expression profiles and histology of liver, epididymal adipose tissue and colon. Lipase activity, fecal lipid profiles and plasma metabolomic analysis were assessed. Fecal 16s rRNA sequencing was obtained and selected bacterial species were used for in vitro studies. Fecal microbiota transplantation and monocolonization were conducted to antibiotic-treated or germ-free (GF) mice.
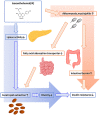
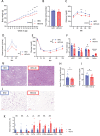
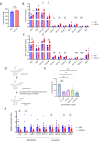
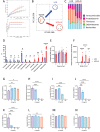
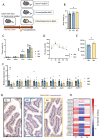
Results: The administration of IX lowered weight gain, decreased steatohepatitis and improved glucose metabolism. Mechanistically, IX inhibited pancreatic lipase activity and lipid absorption by decreasing the expression of the fatty acid transporter CD36 in the small intestine, which was confirmed by increased lipid excretion in feces. IX administration increased markers of intestinal barrier function, including thickening the mucin layer and increasing caludin-1, a tight-junction related protein in the colon. In contrast, the effects of IX were nullified by antibiotics. As revealed using 16S rRNA sequencing, the microbial community structure changed with a significant increase in the abundance of Akkermansia muciniphila in the IX-treated group. An anaerobic chamber study showed that IX selectively promoted the growth of A. muciniphila while exhibiting antimicrobial activity against some Bacteroides and Clostridium species. To further explore the direct effect of A. muciniphila on lipid and glucose metabolism, we monocolonized either A. muciniphila or Bacteroides thetaiotaomicron to GF mice. A. muciniphila monocolonization decreased CD36 expression in the jejunum and improved glucose metabolism, with decreased levels of multiple classes of fatty acids determined using plasma metabolomic analysis.
Conclusions: Our study demonstrated that IX prevents obesity and enhances glucose metabolism by inhibiting dietary fat absorption. This mechanism is linked to suppressing pancreatic lipase activity and shifts in microbial composition, notably an increase in A. muciniphila. These highlight new treatment strategies for preventing metabolic syndrome by boosting the gut microbiota with food components.
Keywords: Akkermansia muciniphila; Insulin resistance; Isoxanthohumol; Lipid absorption; Microbiota; Obesity.
Copyright © 2023 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Koh A., Backhed F. From association to causality: the role of the gut microbiota and its functional products on host metabolism. Mol Cell. 2020;78(4):584–596. - PubMed
-
- Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. - PubMed
-
- Kimura I., Ichimura A., Ohue-Kitano R., Igarashi M. Free fatty acid receptors in health and disease. Physiol Rev. 2020;100(1):171–210. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

